Novel orthoester crosslinking agent monomer and method using same to prepare acid-sensitive nano drug carrier
A nano-drug carrier and cross-linking agent technology, which can be used in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of reducing drug efficacy, achieve good biocompatibility, prolong internal delivery time, and enhance tumor targeting. tropism effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
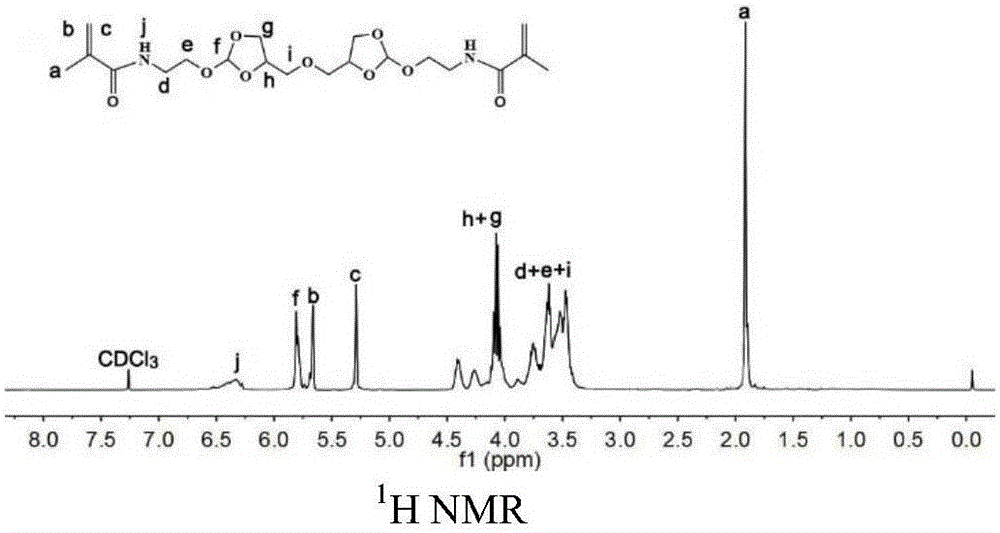
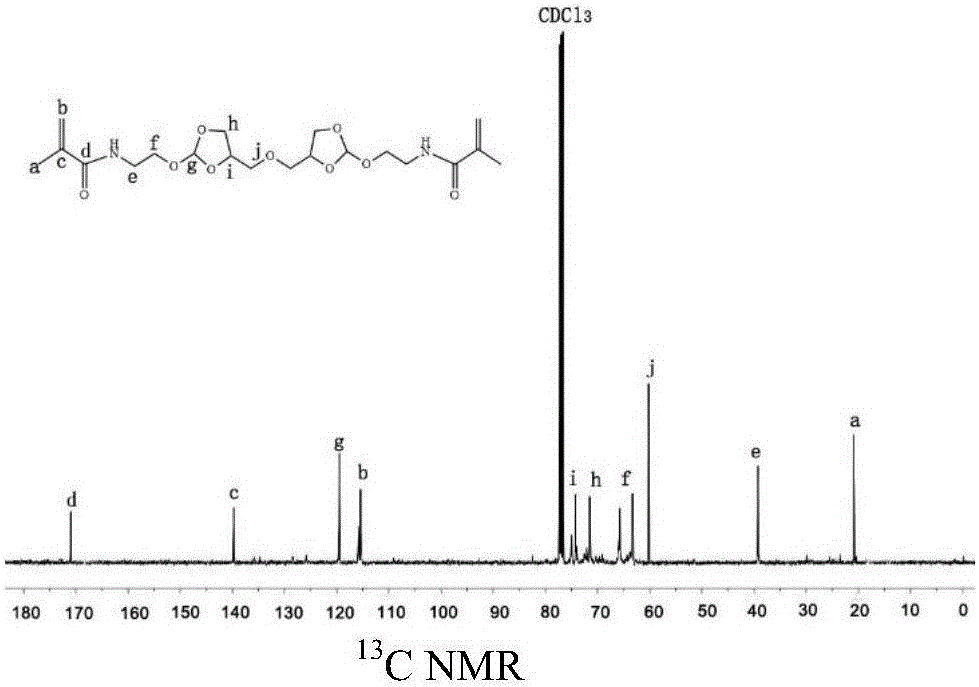
[0032] Preparation of N'N-{2-[2-methoxy-[1,3]dioxolane-4-methyleneoxy)]-ethyl}-2-methacrylamide
[0033] Under nitrogen atmosphere, add orthoester diamine monomer 5.59g (18.1mmol) triethylamine 5.49g (54mmol) and 100ml dichloromethane in the three-necked flask of 250ml, after stirring and dissolving, methacrylic anhydride 5.58g (36.2 mmol) was added dropwise to the above mixture, and after overnight reaction at room temperature, the crude product was separated by silica gel column chromatography to obtain 5.06 g of a yellow oily product with a yield of 53%. 1 H NMR (400MHz, CDC1 3 ): δ(ppm)1.97(s,6H,C-CH 3 ),3.32-3.33(m,3H,O-CH 3 ),3.48-3.75(m,8H,CHZ-O-CH 2 ,O-CH 2 -CH 2 -NH),3.77-4.2(m,4H,CH-O-CH 2 ),4.28-4.51(m,2H,O-CH-CH 2 ),5.34-5.36(m,2H,C=CH 2 ),5.71-5.76(m,2H,CH-(O) 3 ),5.84-5.86(m,2H,C=CH 2 ). 3 CNMR (400MHz, CDC1 3 ,8):18.49,39.33,42.37,61.53,65.64,66.02,71.62,72.5574.05,75.02,115.40,115.89,119.81,139.70,168.49. 20 h 32 N 2 o 9 ),444.21,found m / z,443.2...
Embodiment 2
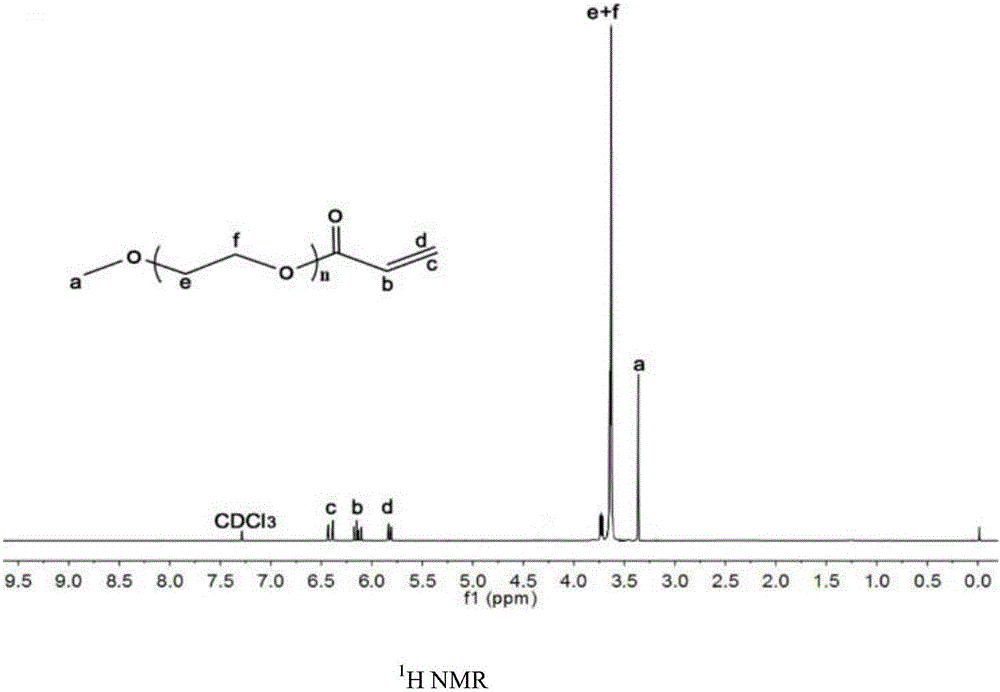
[0035] Preparation of polyethylene glycol monomethyl ether acrylamide
[0036]Under a nitrogen atmosphere, dissolve 5.28g (9.6mmol) of polyethylene glycol monomethyl ether and 2.9g (28.8mmol) of triethylamine into a 250ml three-necked flask. After stirring and dissolving, add 2.6g (28.8mmol) of acryloyl chloride dropwise Added to the above solution, after overnight reaction at room temperature. Remove a small amount of triethylamine hydrochloride by filtration, add dichloromethane to dilute the reaction solution, use 0.5M HCl, 5% NaCHO 3 Wash the organic phase with saturated NaCl solution, dry the organic phase with anhydrous magnesium sulfate, filter, and remove the organic solvent under reduced pressure to obtain 5.53 g of viscous liquid with a yield of 86.2%. 1 H NMR (400MHz, CDC1 3 )δ(ppm): 3.24(s,3H,PEG-OCH 3 ), 3.51(s, MPEG), 5.76(s, 1H, C=CH 2 ),6.05(s,1H,CH),6.30(s,1H,C=CH 2 ).
Embodiment 3
[0038] Preparation of Acid Sensitive Nano Drug Carriers
[0039] N'N-{2-[2-methoxy-[1,3]dioxolane-4-methyleneoxy)]-ethyl}-2-methacrylamide, polyethylene glycol mono Methyl ether acrylamide, sodium lauryl sulfate and ultrapure water were added into a one-necked flask, and stirring was started to dissolve completely. Nitrogen gas was introduced under the liquid surface to exclude air, the temperature of the oil bath was raised to 70°C and nitrogen gas was continuously introduced, and potassium persulfate was added to initiate polymerization, the stirring speed was adjusted, and the reaction was terminated after 5 hours of reaction. After the reaction solution is cooled, it is loaded into a dialysis bag and dialyzed in ultrapure water, and then freeze-dried to obtain a nano drug carrier. Nano-drug carriers with controllable particle size can be prepared by changing the amount of cross-linking agent, the results are shown in the attached Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com