Kidney bean epoxide hydrolase mutant with improved catalytic activity and enantio-convergent property
An epoxide and hydrolase technology, applied in the fields of genetic engineering and protein expression, can solve the problems of high randomness, large screening workload, and unsatisfactory ee value, and achieve high enantionormality, high catalytic activity, The effect of large application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The construction of embodiment 1 mutant enzyme gene and its expression plasmid
[0020] The amplified nucleotide sequence was the gene pveh1 shown in SEQ ID NO.4, the amplified product was ligated with pUCm-T, transformed into E.coli JM109, and screened by blue and white spots, identified by SacI enzyme digestion and DNA sequencing. The recombinant plasmid with correct sequencing was named pUCm-T-pveh1. Digest pUCm-T-pveh1 with NdeI and XhoI, recover pveh1, and connect it with pET-28a(+) which has been digested with the same double enzymes, to obtain recombinant plasmid pET-28a(+)-pveh1.
[0021] Using the known crystal structure of potato EH (PDB: 2CJP) as a template, the three-dimensional structure of PvEH1 was obtained by homology modeling. The model substrate molecule (R)-SO was molecularly docked with PvEH1 using AutoDock4.2 software, and the catalytic (R)-SO molecule was counted The homology analysis excludes the amino acids at the conserved sites; the types an...
Embodiment 2
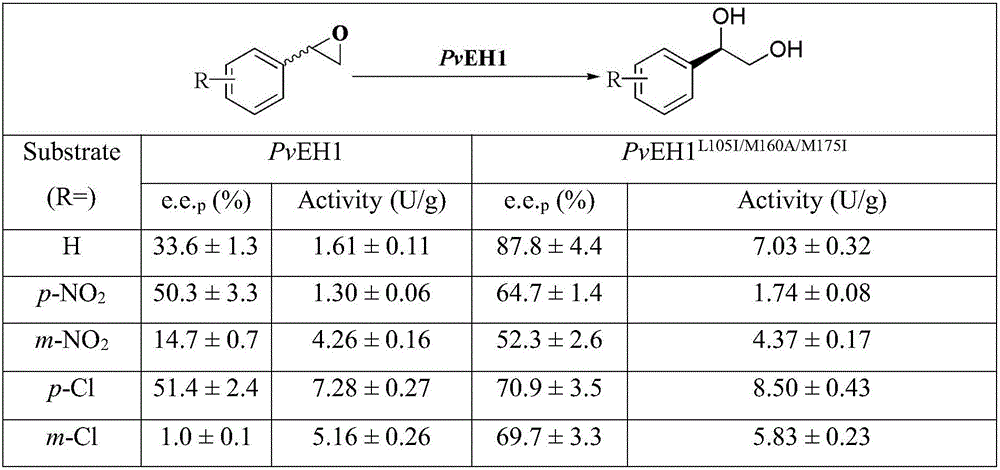
[0030] Example 2 Mutant enzyme PvEH1 L105I / M160A / M175I Determination of catalytic activity
[0031] Add 450 μL of bacterial suspension to a 2 mL EP tube, incubate at 25°C for 5 min, then add 50 μL of racemic styrene oxide (rac-SO, final concentration 20 mmol / L) and react immediately for 10 min, then take 200 μL of the reaction solution and add 1 mL of methanol was used to stop the reaction. After microfiltration, samples were analyzed by reverse phase HPLC (Waters, Milford, MA), C18 column and UV detector. The mobile phase was methanol / water (70:30, v / v), the flow rate was 0.8 mL / min, and the detection wavelength was 220 nm. Definition of enzyme activity unit: Under the conditions of this assay, the amount of enzyme required to decompose 1 μmol rac-SO per minute is defined as the activity unit (IU) of 1 epoxide hydrolase. Mutant enzyme PvEH1 L105I / M160A / M175I The catalytic activity was 10.66U / g, which was 5.6 times higher than that of the wild-type enzyme (1.61U / g).
Embodiment 3
[0032] The mensuration of embodiment 3 enantiomeric purity and enantioselectivity
[0033] Add 450 μL of bacterial suspension and 500 μL of sodium phosphate buffer (pH 7.5) to a 1.5 mL EP tube, incubate at 25 °C for 5 min, then add 50 μL of rac-SO (final concentration 10 mmol / L) for reaction. 50 μL of samples were regularly extracted to 1 mL of ethyl acetate (containing 1 mmol / L n-hexanol as an internal standard) for extraction, and the samples were analyzed using a gas chromatograph GC-2010 (Shimadzu, Japan), a chiral gas chromatography column, and a hydrogen flame ionization detector. The analysis conditions were: inlet and detector temperature 250°C; initial column temperature 100°C, rising to 195°C at 5°C / min; carrier gas nitrogen, flow rate 3.0mL / min, split ratio 1:50. n-Hexanol, (R)-SO((R)-Ethylene Oxide), (S)-SO((S)-Ethylene Oxide), (S)-PED((S)-Phenylethylene Glycol) and (R)-PED ((R)-phenylethylene glycol) retention times were 3.477, 5.959, 6.065, 16.752 and 16.866min,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com