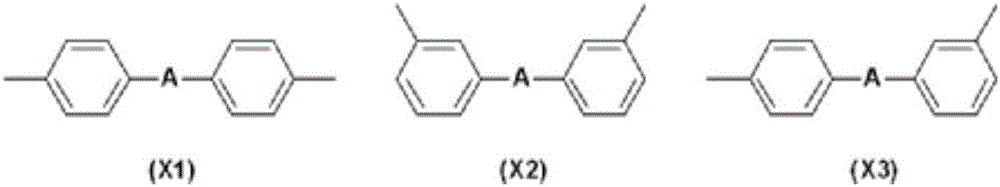
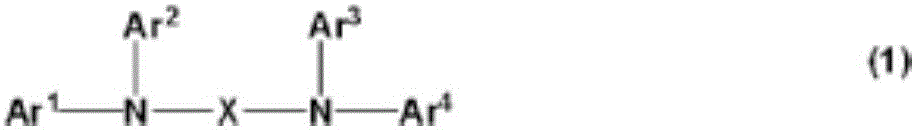Triarylamine derivative and use of same
A technology of triarylamine and its derivatives, which is applied in the field of triarylamine derivatives and their utilization, can solve the problems that triarylamine derivatives have not been reported, and achieve the effect of excellent brightness characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] [Example 1] Synthesis of Triarylamine Derivative A
[0247] According to the following reaction formula, the triarylamine derivative A represented by the formula (1-31) was synthesized.
[0248] [chemical 15]
[0249]
[0250] 15.0 g of 4,4'-(perfluoropropane-2,2-diyl)diphenylamine, 0.52 g of bis(benzylideneacetone)palladium, and 19.0 g of sodium tert-butoxide were added to the flask, and after nitrogen replacement, the To this was added 260 mL of toluene, 21.0 mL of bromobenzene, and 2.60 mL of a toluene solution of tri-tert-butylphosphine prepared in advance (concentration: 0.143 g / mL), and stirred at 50° C. for 2 hours.
[0251] After the stirring, the reaction mixture was left to cool to room temperature, and the cooled mixture was filtered. Then, a liquid separation process was performed using the filtrate and saturated saline.
[0252] Next, the obtained organic layer was dried over sodium sulfate, activated carbon was added to the dried organic layer, and t...
Embodiment 2-1
[0257] Dissolve 0.106 g of oligoaniline derivative represented by formula (f) as a charge-transporting substance and 0.636 g of phosphotungstic acid as a dopant in 1,3-dimethyl-2-imidazolidinone in a nitrogen atmosphere 8g. To the obtained solution, 12 g of cyclohexanol and 4 g of propylene glycol were added and stirred, and 0.074 g of triarylamine derivative A was added thereto and stirred again to prepare a charge-transporting varnish. In addition, the oligoaniline derivative represented by formula (f) was synthesize|combined by the method of International Publication No. 2013 / 084664.
[0258] [chemical 16]
[0259]
Embodiment 3-1
[0264] The varnish prepared in Example 2-1 was coated on the ITO substrate using a spin coater, then dried at 50°C for 5 minutes, and then baked at 230°C for 15 minutes in the atmosphere, and then coated on the ITO substrate A uniform thin film of 30 nm was formed on it. As the ITO substrate, a glass substrate of 25 mm × 25 mm × 0.7 t, which was patterned with indium tin oxide (ITO) on the surface with a film thickness of 150 nm, was used before use. 2 A plasma scrubber (150W, 30 seconds) removes impurities on the surface.
[0265] Next, use a vapor deposition device (vacuum degree 1.0×10 -5 Pa), a thin film of α-NPD and aluminum was sequentially laminated on the ITO substrate on which the thin film was formed, to obtain a two-layer element. At this time, both α-NPD and aluminum were vapor-deposited at the respective vapor-deposition rates of 0.2 nm / sec, and the film thicknesses were 30 nm and 100 nm, respectively.
[0266] In addition, in order to prevent deterioration of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com