Multi-conduction-site polyphenyl ether based anion-exchange membrane for fuel cells and preparation method of multi-conduction-site polyphenyl ether based anion-exchange membrane
An anion exchange membrane and fuel cell technology, which is applied in the field of fuel cell materials and anion exchange membranes, can solve the problems of reducing fuel cell performance, increasing fuel cell cost, and easy CO poisoning of catalysts, so as to maintain relative integrity and dimensional stability Good, the effect of simplifying the synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
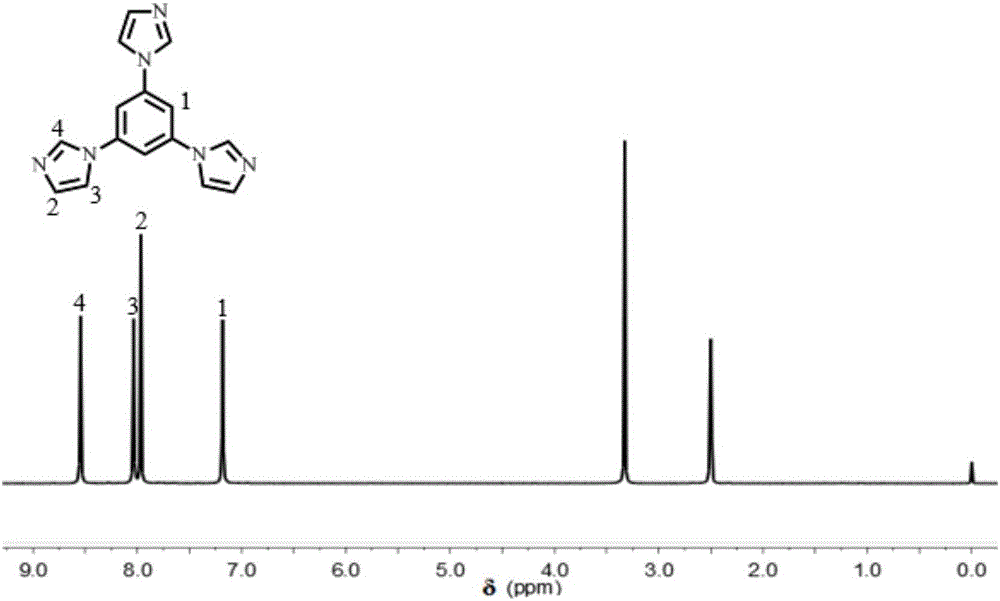
[0051] 1. Preparation of polyphenylene ether (PPO) nitromethylimidazole skeleton:
[0052] Add 0.3g of commercially available polyphenylene ether to a 100mL three-necked flask equipped with a stirrer, condenser and air duct. Heat to 50°C under a nitrogen atmosphere. After the polyphenylene ether is completely dissolved, add 4.64g of commercially available peroxide Benzoyl (PPO) and 0.232g of commercially available N-bromosuccinimide (NBS), the temperature of the oil bath was raised to 80°C, after 4 hours of reaction, the temperature was lowered to room temperature, and the reactant solution was transferred In a rotary steaming flask, at 60°C, rotary steam until the color of the solution becomes brown, and then precipitate in methanol to obtain a light yellow solid. Dissolve the light yellow solid with commercially available dichloromethane, rotary steam and precipitate, and repeat this Operate 3 times to obtain pure light yellow brominated polyphenylene ether (BPPO), dry in an ov...
Embodiment 2
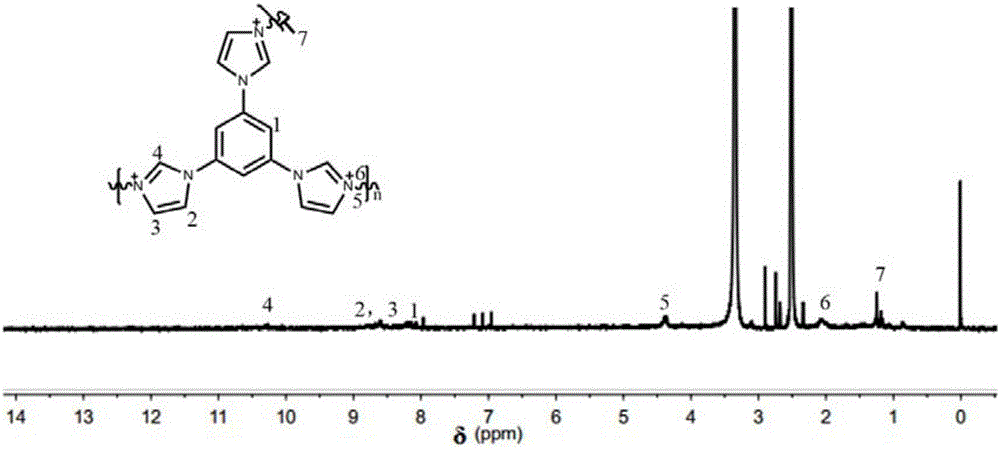
[0064] 1. Preparation of polyphenylene ether (PPO) nitromethylimidazole skeleton:
[0065] Add 0.3g of commercially available polyphenylene ether to a 100mL three-necked flask equipped with a stirrer, condenser and air duct. Heat to 50°C under a nitrogen atmosphere. After the polyphenylene ether is completely dissolved, add 4.64g of commercially available peroxide Benzoyl (PPO) and 0.232g of commercially available N-bromosuccinimide (NBS), raise the temperature of the oil bath to 80°C for 4 hours, then lower the temperature to room temperature, and transfer the reactant solution to In a rotary steaming flask, rotary steam at 60°C until the solution turns brown, and then precipitate in methanol to obtain a light yellow solid. Use commercially available dichloromethane to dissolve the light yellow solid, rotary steam, and precipitate. Repeat this operation 3 times. Obtain pure light yellow brominated polyphenylene ether (BPPO), dry it in an oven at 60°C, dissolve 0.25 g of dried BP...
Embodiment 3
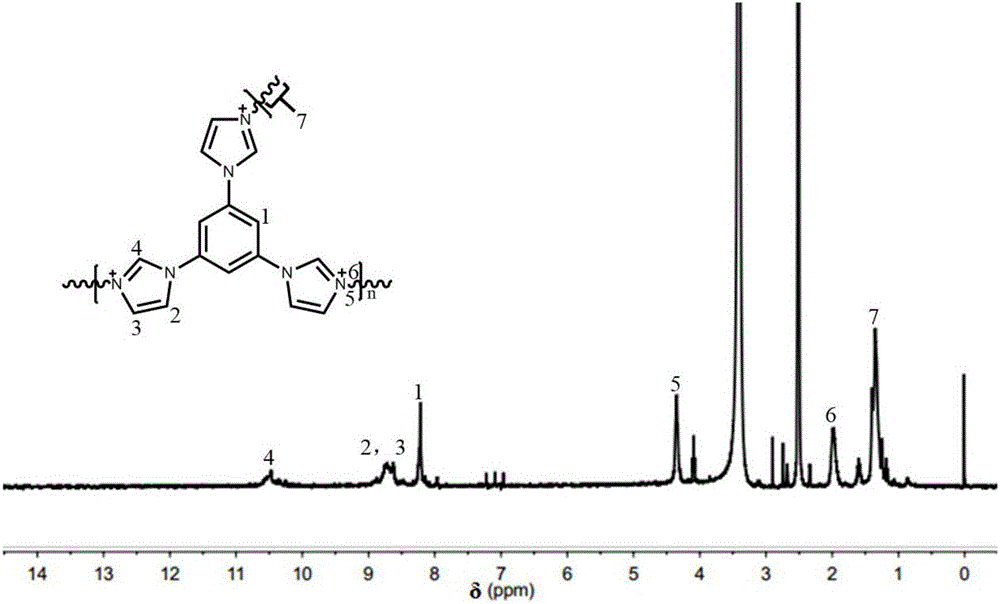
[0076] 1. Preparation of polyphenylene ether (PPO) nitromethylimidazole skeleton:
[0077] Add 0.3g of commercially available polyphenylene ether to a 100mL three-necked flask equipped with a stirrer, condenser and air duct. Heat to 50°C under a nitrogen atmosphere. After the polyphenylene ether is completely dissolved, add 4.64g of commercially available peroxide Benzoyl (PPO) and 0.232g of commercially available N-bromosuccinimide (NBS), raise the temperature of the oil bath to 80°C for 4 hours, then lower the temperature to room temperature, and transfer the reactant solution to In a rotary steaming flask, rotary steam at 60°C until the solution is brown, and then precipitate in methanol to obtain a light yellow solid. Use commercially available dichloromethane to dissolve the light yellow solid, rotary steam, and precipitate. Repeat this operation 3 times to obtain Pure light yellow brominated polyphenylene oxide (BPPO), dried in an oven at 60°C, dissolve 0.25 g of dried BPPO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com