Antibody-modified fluorescent nanoparticles and application thereof in targeted imaging of cancer cells
A fluorescent nanometer and antibody modification technology, which is applied in the field of medical materials, can solve problems such as quenching and self-quenching of fluorescence, and achieve the effects of high photostability, low cytotoxicity and specific recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A method for preparing antibody-modified fluorescent nanoparticles, comprising the following steps:
[0048] Dissolve distearoylphosphatidylethanolamine-polyethylene glycol-2000 (1mg), distearoylphosphatidylethanolamine-polyethylene glycol-2000-carboxy (1mg), fluorophore t-BuPITBT-TPE (1mg) In 1 mL of tetrahydrofuran, 9 mL of distilled water was added under ultrasound (80% output, SCIENTZ-II D ultrasonic instrument), and fluorescent nanoparticles surface-modified with maleic anhydride were directly prepared by nanoprecipitation method. Blow nitrogen to the liquid surface for 2 hours at room temperature to remove tetrahydrofuran through volatilization, and further filter through a 0.2 μm filter head to remove precipitates and large particles. Add cetuximab (80 μL, 5 mg / mL), N-hydroxysulfosuccinimide (17.4 μg) and 1-(3-dimethylaminopropyl)-3-ethylcarbodisulfate to the filtrate Imine hydrochloride (15.3 μg), reacted at room temperature for 4 hours, removed excess small mo...
Embodiment 2
[0050] The measurement of the fluorescent nanoparticle concentration that embodiment 1 makes
[0051] Different concentrations of t-BuPITBT-TPE were dissolved in THF solution, and the standard curve corresponding to its absorption intensity at 475nm and concentration was obtained by detecting its ultraviolet-visible light absorption at different concentrations.
[0052] After freeze-drying 1mL t-BuPITBT-TPE-C225 nanoparticle solution, dissolve it in 3mL THF, measure its absorption value at 475nm, and calculate the t-BuPITBT- The TPE concentration was 18.73 μg / mL, which was used in the following cell experiments.
Embodiment 3
[0054] The particle size and the photophysical property of the fluorescent nanoparticle that embodiment 1 makes
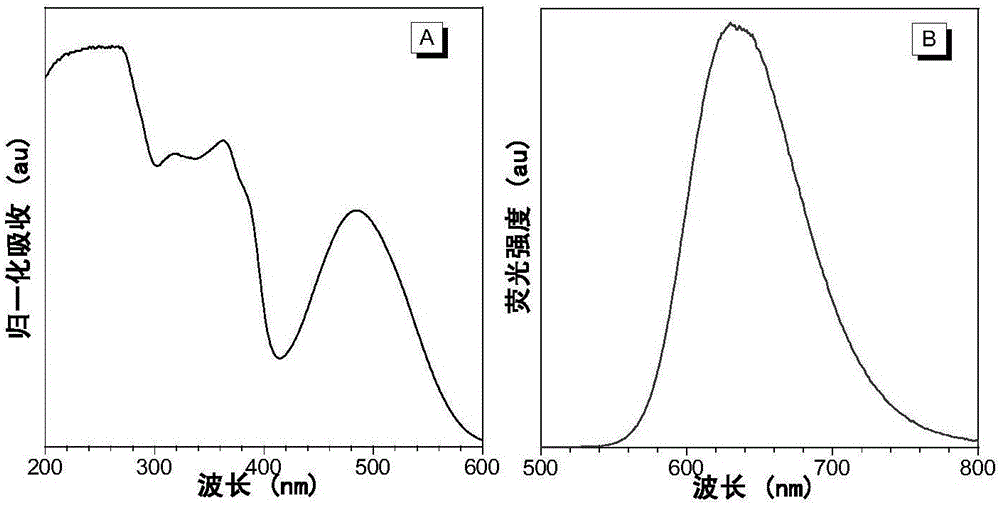
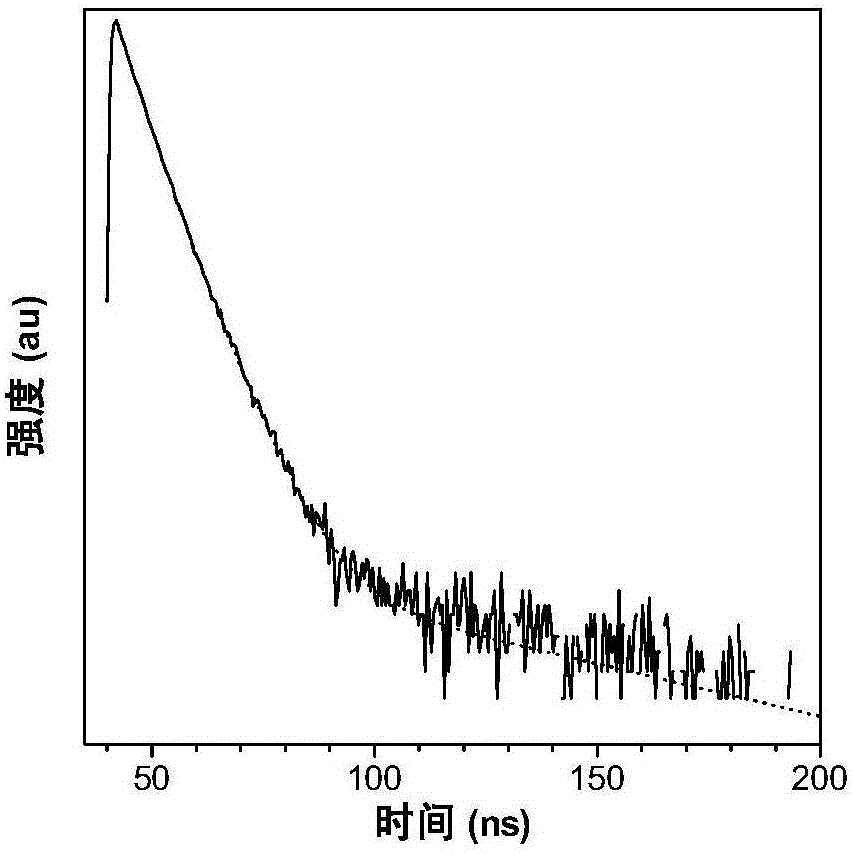
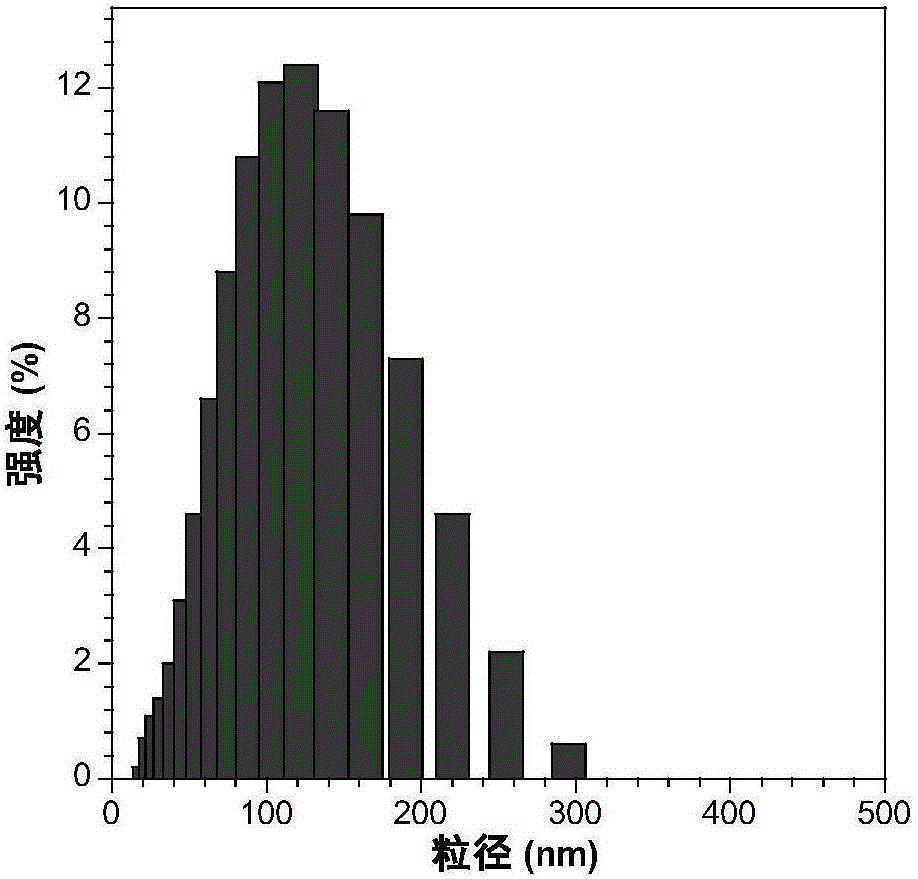
[0055] The nanoparticle t-BuPITBT-TPE-C225 has a maximum absorption at 475nm and a maximum emission at 625nm ( figure 1 ). The quantum yield is 35.1%, and the fluorescence lifetime is 4.63ns ( figure 2 ). The hydrated particle size of t-BuPITBT-TPE nanoparticles is 97nm, and the degree of dispersion (PDI) is 0.19 ( image 3 , measured by dynamic light scattering); the hydrated particle size of the t-BuPITBT-TPE-C225 nanoparticles modified with cetuximab is 116nm, and the degree of dispersion (PDI) is 0.33 ( Figure 4 ). It was further confirmed by TEM that the prepared fluorescent nanoparticles had good dispersion in aqueous solution ( Figure 5 and Figure 6 , Figure 5 was measured by dynamic light scattering).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com