Preparation method of layered lithium-rich positive electrode material
A lithium-rich positive electrode material and solvothermal technology, applied in battery electrodes, electrical components, electrochemical generators, etc., can solve the problems of high irritation of ammonia water, difficulty in precise control of stable pH value, and large product fluctuations. Achieve the effects of simple process, easy control of shape and size, and excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
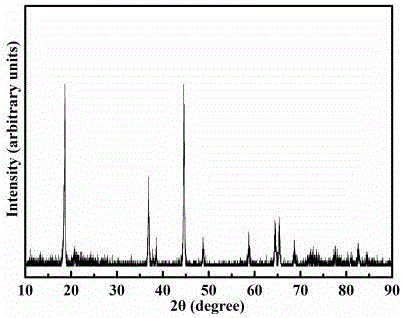
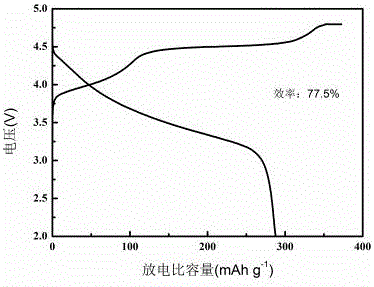
Image
Examples
Embodiment 1
[0032] Weigh 1.6230g of lithium acetate dihydrate, 0.4232g of nickel acetate tetrahydrate, 1.6500g of manganese acetate tetrahydrate, and 0.4172g of cobalt acetate tetrahydrate and dissolve them in 40mL of ethanol, stir until completely dissolved, and dissolve 40mL of oxalic acid 4.4124g The ethanol solution was poured slowly for co-precipitation. The resulting suspension was transferred to a reaction kettle, and solvothermally reacted at 180°C for 24 hours. After natural cooling, the product was washed and filtered with alcohol, and the precipitate was dried at 100°C for 10 hours. The obtained sample was pre-calcined in air at 450°C for 5 hour, and then the sample was calcined at 900°C for 12 hours, and the obtained powder was the layered lithium-rich cathode material.
[0033] Battery assembly: Weigh 0.4g of the obtained layered lithium-rich cathode material, add 0.05g of conductive carbon black (Super-P) as a conductive agent and 0.05g of PVDF (HSV900) as a binder, and add ...
Embodiment 2
[0035]Weigh 1.6230g of lithium acetate dihydrate, 0.4232g of nickel acetate tetrahydrate, 1.6500g of manganese acetate tetrahydrate, and 0.4172g of cobalt acetate tetrahydrate and dissolve them in 40mL of ethanol, stir until completely dissolved, and dissolve 40mL of oxalic acid 6.6186g The ethanol solution was poured slowly for co-precipitation. The obtained suspension was transferred to a reaction kettle, and solvothermally reacted at 180°C for 24 hours. After natural cooling, the product was washed and filtered with alcohol, and the precipitate was dried at 100°C for 10 hours. The obtained sample was pre-calcined in air at 450°C for 5 hour, and then the sample was calcined at 900°C for 12 hours, and the obtained powder was the layered lithium-rich cathode material. The sample was prepared and assembled into a CR2025 button battery, and the specific capacity of the first discharge at 0.1C was 265mAh / g.
Embodiment 3
[0037] Weigh 1.7004g of lithium acetate dihydrate, 0.4232g of nickel acetate tetrahydrate, 1.6500g of manganese acetate tetrahydrate, and 0.4172g of cobalt acetate tetrahydrate and dissolve them in 40mL of ethanol, stir until completely dissolved, and dissolve 40mL of oxalic acid 5.5155g The ethanol solution was poured slowly for co-precipitation. The obtained suspension was transferred to a reaction kettle, and solvothermally reacted at 180°C for 24 hours. After natural cooling, the product was washed and filtered with alcohol, and the precipitate was dried at 100°C for 10 hours. The obtained sample was pre-calcined in air at 450°C for 5 hour, and then the sample was calcined at 900°C for 12 hours, and the obtained powder was the layered lithium-rich cathode material. The sample was prepared and assembled into a CR2025 button battery, and the specific capacity of the first discharge at 0.1C was 231.6mAh / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Stress tolerance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com