Solid preparation of epalrestat solid dispersion and preparation method thereof
A technology of solid dispersion and epalrestat, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulations, etc., which can solve the problems of difficult absorption and utilization of preparations, low in vitro dissolution rate, Poor solubility and other problems, to achieve the effect of improving bioavailability, easy preparation process, and accelerated dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
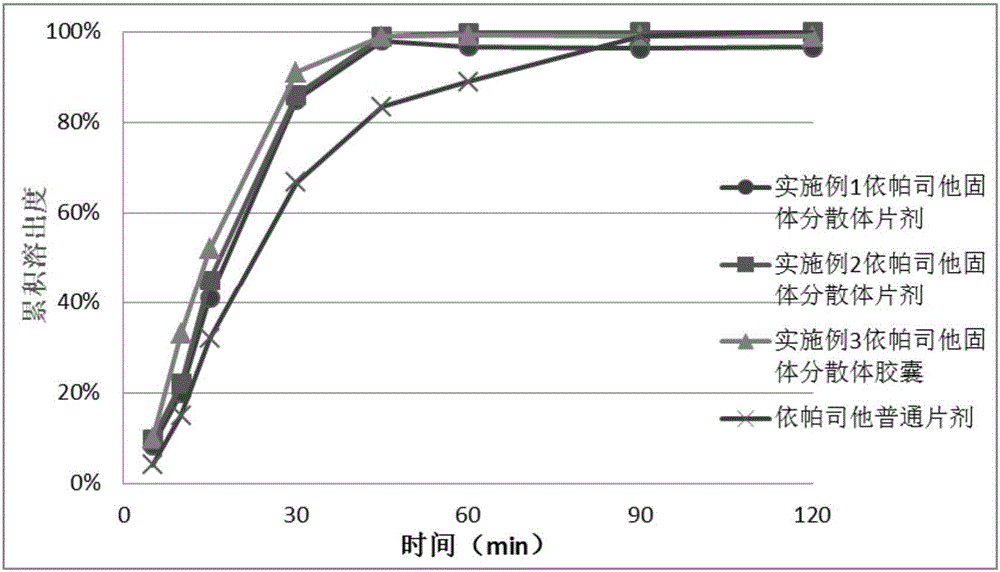
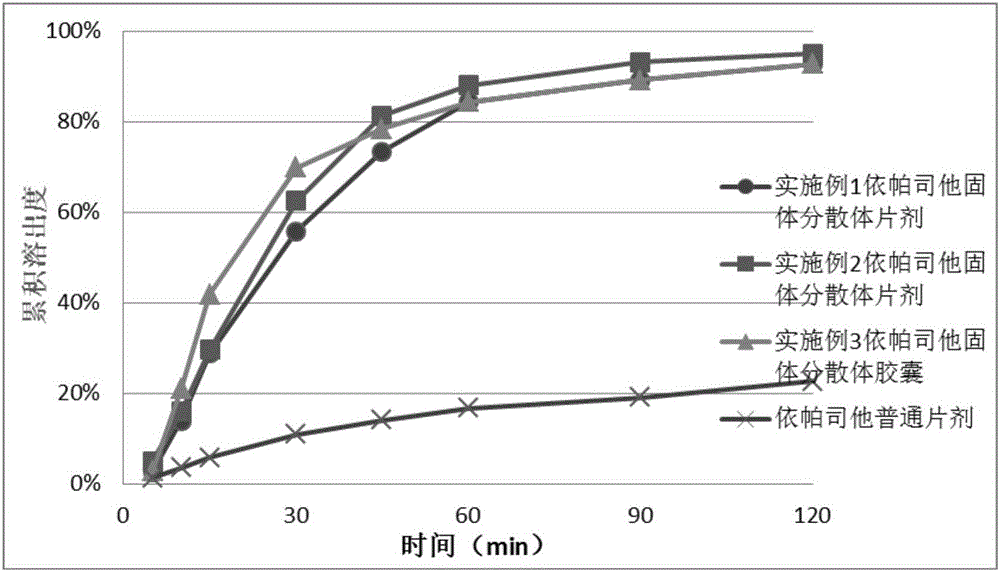
Image
Examples
Embodiment 1
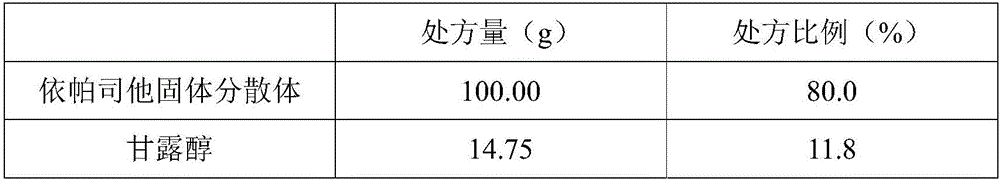
[0033] The solid preparation of the epalrestat solid dispersion of the present embodiment is made up of epalrestat solid dispersion and auxiliary materials, wherein said epalrestat solid dispersion is made up of epalrestat raw material, solubilizer meglumine and carrier material PEG-4000 and PEG-6000 are composed according to the weight ratio of 1:0.5:0.5; the excipients include filler mannitol, disintegrant low-substituted hydroxypropyl cellulose and sodium carboxymethyl starch, binder hydroxypropyl methyl base cellulose and glidant micropowder silica gel; the ratio of epalrestat solid dispersion and auxiliary materials is according to weight: 100 parts of solid dispersion, 14.75 parts of filler, 7.5 parts of disintegrant, 2.5 parts of binder, auxiliary 0.25 part of liquid agent, as shown in table 1:
[0034] Table 1 The prescription ratio of epalrestat solid dispersion and auxiliary materials (specification 50mg, 1000 tablets)
[0035]
[0036]
[0037] The preparatio...
Embodiment 2
[0042] The solid preparation of the epalrestat solid dispersion of the present embodiment is made up of epalrestat solid dispersion and auxiliary materials, wherein said epalrestat solid dispersion is made up of epalrestat raw material, solubilizer meglumine and carrier material PVPK30 is composed according to the weight ratio of 1:5:5; the excipients include filler microcrystalline cellulose, disintegrant low-substituted hydroxypropyl cellulose, binder hydroxypropyl cellulose and glidant talc; The ratio of other solid dispersions and auxiliary materials is shown in Table 2:
[0043] Table 2 The prescription ratio of epalrestat solid dispersion and auxiliary materials (specification 50mg, 1000 tablets)
[0044]
Prescription volume (g)
Prescription ratio (%)
Epalrestat solid dispersion
550.00
89.4
17.65
2.9
Low-substituted hydroxypropyl cellulose
33.83
5.5
12.30
2.0
...
Embodiment 3
[0050] The solid preparation of the epalrestat solid dispersion of the present embodiment is made up of epalrestat solid dispersion and auxiliary materials, wherein said epalrestat solid dispersion is made up of epalrestat raw material, solubilizer meglumine and carrier material Mannitol is composed according to the weight ratio of 1:0.2:10; the auxiliary materials include filler lactose, binder hydroxypropyl methylcellulose, disintegrant cross-linked polyvinylpyrrolidone and glidant micropowder silica gel; The ratio of other solid dispersions and auxiliary materials is shown in Table 3:
[0051] Table 3 The prescription ratio of epalrestat solid dispersion and excipients (specification 50mg, 1000 capsules)
[0052]
Prescription volume (g)
Prescription ratio (%)
Epalrestat solid dispersion
560.00
92.6
13.54
2.2
Hydroxypropylmethylcellulose
18.15
3.0
Cross-linked polyvinylpyrrolidone
12.10
2.0
M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com