Method for synthesizing acridone derivatives by means of palladium-copper co-catalysis
A copper co-catalyzed synthesis of acridone and derivatives, which is applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., achieving the effects of large application potential, high atom economy, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
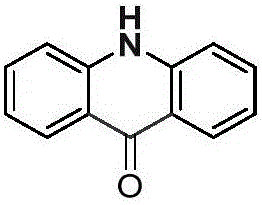
[0019] Palladium chloride (0.025mmol), copper pivalate (0.05mmol), and diphenylamine (0.25mmol) were added to a dry Schlenk reaction tube, and the system was replaced three times under an atmospheric pressure of carbon monoxide to obtain a pure carbon monoxide atmosphere. Then add solvent anhydrous dimethyl sulfoxide (1.0mL), DTBP (0.75mmol) and solvent anhydrous dimethyl sulfoxide (2.0mL) successively, stop the reaction after reacting at 100°C for 24 hours, cool to room temperature, and Ethyl acetate was added to the reaction system to quench the reaction, and saturated potassium carbonate solution (30.0 mL) was added, extracted with dichloromethane (20×3), separated by column chromatography to obtain an acridone derivative, and the separation yield reached 85%. The NMR characterization data are as follows:
[0020]
[0021] NMR data: 1 H NMR (400MHz, DMSO-d 6 )δ=11.77(s,1H),8.25(d,J=7.7,2H),7.74(t,J=7.1,2H),7.56(d,J=8.1,2H),7.26(t,J=7.1 ,2H). 13 C NMR (101MHz, DMSO-d ...
Embodiment 2
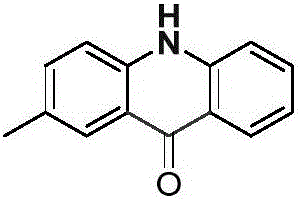
[0023] Palladium chloride (0.025mmol), copper pivalate (0.05mmol) and p-methyldiphenylamine (0.25mmol) were added to a dry Schlenk reaction tube, and the system was replaced three times under an atmospheric pressure of carbon monoxide. Then add solvent anhydrous dimethyl sulfoxide (1.0mL), DTBP (0.75mmol) and solvent anhydrous dimethyl sulfoxide (2.0mL) successively, stop the reaction after reacting at 100°C for 24 hours, cool to room temperature, and Dichloromethane was added to the reaction system for dilution, and saturated potassium carbonate solution (30.0mL) was added, extracted with dichloromethane (20×3), and separated by column chromatography to obtain acridone derivatives with a separation yield of 79%. NMR The characterization data are as follows:
[0024]
[0025] NMR data: 1 H NMR (400MHz, DMSO-d 6 )δ=11.70(s,1H),8.22(d,J=8.1,1H),8.02(s,1H),7.71(t,J=7.7,1H),7.59–7.44(m,3H),7.23( t,J=7.5,1H),2.41(s,3H). 13 C NMR (101MHz, DMSO-d 6 ) δ = 177.0, 141.2, 139.4, ...
Embodiment 3
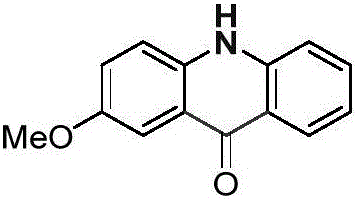
[0027] Palladium chloride (0.025mmol), copper pivalate (0.05mmol) and p-methoxydiphenylamine (0.25mmol) were added into a dry Schlenk reaction tube, and the system was replaced three times under an atmospheric pressure of carbon monoxide. Then add solvent anhydrous dimethyl sulfoxide (1.0mL), DTBP (0.75mmol) and solvent anhydrous dimethyl sulfoxide (2.0mL) successively, stop the reaction after reacting at 100°C for 24 hours, cool to room temperature, and Ethyl acetate was added to the reaction system to quench the reaction, and saturated potassium carbonate solution (30.0 mL) was added, extracted with dichloromethane (20×3), separated by column chromatography to obtain an acridone derivative, and the separation yield reached 81%. The NMR characterization data are as follows:
[0028]
[0029] NMR data: 1 H NMR (400MHz, DMSO-d 6 )δ=11.78(s,1H),8.24(d,J=8.1,1H),7.71(t,J=7.7,1H),7.64(d,J=2.9,1H),7.55(d,J=3.1 ,1H),7.53(d,J=2.2,1H),7.42(dd,J=9.0,3.0,1H),7.24(t,J=7.5,1H),3.87(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com