Erythromycin ethylsuccinate tablet and preparation method thereof
A technology of erythromycin ethylsuccinate tablets and erythromycin ethylsuccinate, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of reducing the type and amount of excipients, tablet Complicated preparation process, poor product dissolution, etc., to avoid the increase of related substances, improve stability, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
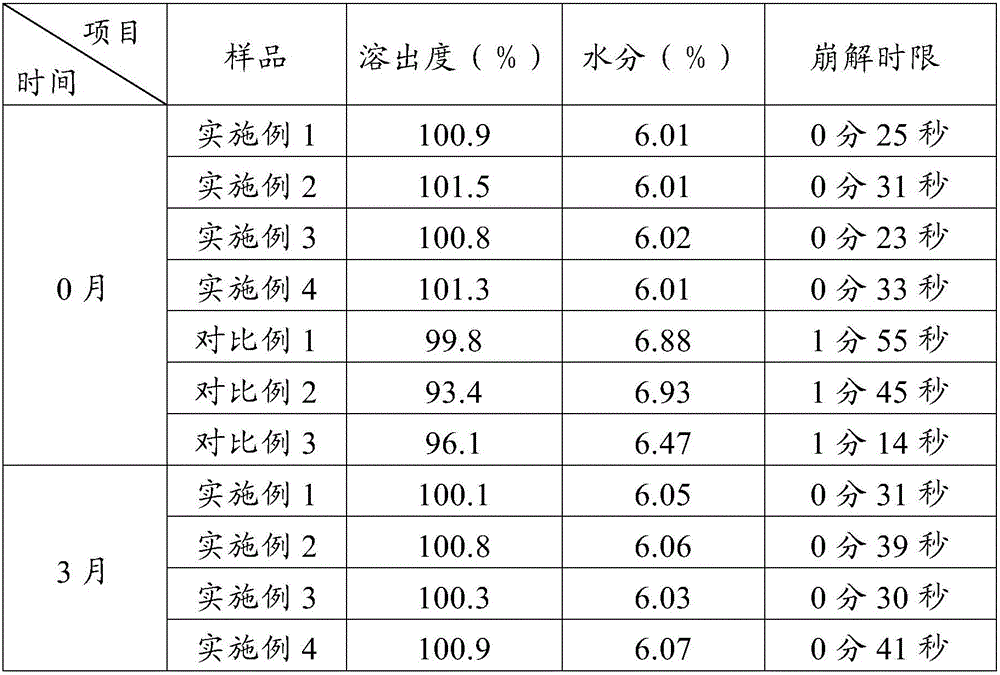
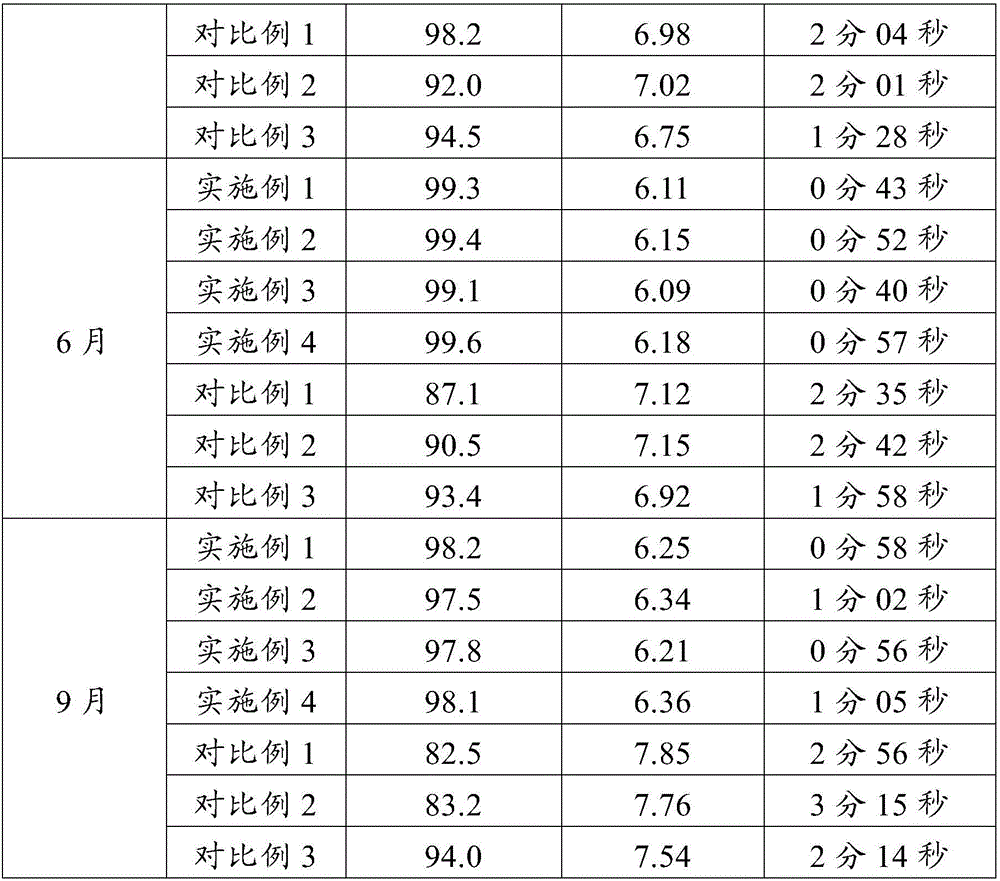
Embodiment 1
[0057] Prescription: based on 1000 tablets
[0058] erythromycin ethylsuccinate 125g starch 100g
[0059] Carboxymethyl Starch Sodium 12g Microcrystalline Cellulose 6g
[0060] Hydroxypropyl Cellulose 6g Magnesium Stearate 6g
[0061] Preparation:
[0062] (1) Sieving: pass erythromycin ethylsuccinate through an 80-mesh sieve, and other auxiliary materials through a 100-mesh sieve;
[0063] (2) Granulation: Take starch and microcrystalline cellulose and place them in a rotary granulator, spray 50 g of hydroxypropyl cellulose aqueous solution with a mass fraction of 4%, granulate, and make 40-mesh granules. Dry for 2 hours as empty granules; take the empty granules and place them in the granulation chamber of the rotary granulator, and put the pre-crushed erythromycin ethylsuccinate powder in the powder hopper of the granulator, and spray the mass 50 g of hydroxypropyl cellulose aqueous solution with a fraction of 4%, granulate, and dry at 45°C for 2 hours to obtain drug-cont...
Embodiment 2
[0066] Prescription: based on 1000 tablets
[0067] erythromycin ethylsuccinate 125g starch 100g
[0068] Carboxymethyl Starch Sodium 12g Microcrystalline Cellulose 10g
[0069] Hydroxypropyl Cellulose 10g Magnesium Stearate 10g
[0070] Preparation:
[0071] (1) Sieving: pass erythromycin ethylsuccinate through an 80-mesh sieve, and other auxiliary materials through a 100-mesh sieve;
[0072] (2) Granulation: Take starch and microcrystalline cellulose and place them in a rotary granulator, spray 100 g of hydroxypropyl cellulose aqueous solution with a mass fraction of 5%, granulate, and make 41-mesh granules. Dry for 3 hours as empty granules; take the empty granules and place them in the granulation chamber of the rotary granulator, and put the pre-crushed erythromycin ethylsuccinate powder in the powder hopper of the granulator, and spray the mass 50g of hydroxypropyl cellulose aqueous solution with a fraction of 5%, granulate, and dry at 40°C for 3 hours to obtain drug...
Embodiment 3
[0075] Prescription: based on 1000 tablets
[0076] erythromycin ethylsuccinate 100g starch 105g
[0077] Carboxymethyl Starch Sodium 10g Microcrystalline Cellulose 1g
[0078] Hydroxypropyl Cellulose 2g Magnesium Stearate 3g
[0079] Preparation:
[0080] (1) Sieving: pass erythromycin ethylsuccinate through an 80-mesh sieve, and other auxiliary materials through a 100-mesh sieve;
[0081] (2) Granulation: Take starch and microcrystalline cellulose and place them in a rotary granulator, spray 50 g of hydroxypropyl cellulose aqueous solution with a mass fraction of 2%, granulate, and make 40-mesh granules. Dry for 3 hours as empty granules; take the empty granules and place them in the granulation chamber of the rotary granulator, and put the pre-crushed erythromycin ethylsuccinate powder in the powder hopper of the granulator, and spray the mass 50 g of hydroxypropyl cellulose aqueous solution with a fraction of 1%, granulate, and dry at 40°C for 2 hours to obtain drug-co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com