Preparation method of neohesperidin dihydrochalcone
A technology of neohesperidin dihydrochalcone and neohesperidin, which is applied in the field of preparation of neohesperidin dihydrochalcone, can solve the problem of high power consumption, product purity of only 96%, low yield, etc. problem, to achieve the effect of improving utilization, low cost and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
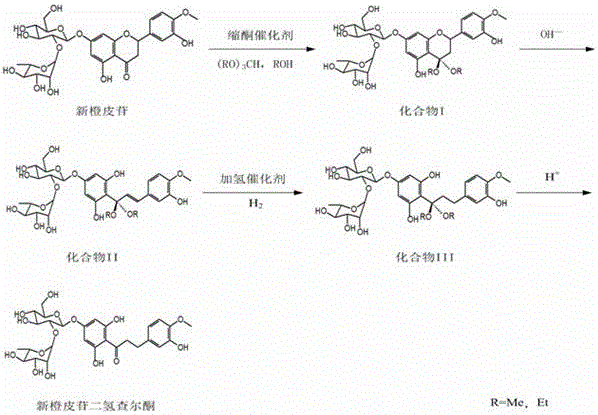
[0033] (1) Carbonyl protection: Dissolve 50g of neohesperidin in 500g of ethanol with a mass concentration of 99%, add 20g of triethyl orthoformate and 2.5g of ketal catalyst lithium tetrafluoroborate, and perform ketalization at 25°C Reacted for 2 hours, concentrated under reduced pressure, cooled and crystallized, filtered and dried to obtain 51.6 g of compound Ⅰ;
[0034] (2) Hydrolysis reaction: 51.6 g of compound I obtained in step (1) was dissolved in 516 g of potassium hydroxide aqueous solution with a mass concentration of 2%, to obtain a potassium hydroxide solution of compound II;
[0035](3) Hydrogenation reaction: Add 5 g of hydrogenation catalyst palladium carbon into the potassium hydroxide solution of compound II obtained in step (2), first pass nitrogen protection into the reaction vessel, and then at a temperature of 25 ° C and a pressure of 0.01 MPa, Pass hydrogen into the potassium hydroxide solution of compound II, carry out the hydrogenation reaction for 1...
Embodiment 2
[0038] (1) Carbonyl protection: Dissolve 100g neohesperidin in 1100g methanol with a mass concentration of 99%, add 45g trimethyl orthoformate and 7.5g ketal catalyst decane boron, and carry out ketal reaction at 65°C 6h, concentrated under reduced pressure, cooled and crystallized, filtered and dried to obtain 103.1g of compound Ⅰ;
[0039] (2) Hydrolysis reaction: 103.1 g of compound I obtained in step (1) was dissolved in 1200 g of 5% sodium carbonate aqueous solution to obtain a sodium carbonate solution of compound II;
[0040] (3) Hydrogenation reaction: Add 15g of hydrogenation catalyst Raney nickel to the sodium carbonate solution of compound II obtained in step (2), first pass nitrogen protection into the reaction vessel, and then at a temperature of 35°C and a pressure of 0.5MPa, Pass hydrogen into the sodium carbonate solution of compound II, carry out the hydrogenation reaction for 20 h, filter and recover the hydrogenation catalyst Raney nickel, and obtain the sod...
Embodiment 3
[0043] (1) Carbonyl protection: Dissolve 100g neohesperidin in 1200g ethanol with a mass concentration of 95%, add 40g triethyl orthoformate and 5g ketal catalyst indium trichloride, and carry out ketal reaction at 70°C 3h, concentrated under reduced pressure, cooled and crystallized, filtered and dried to obtain 102.7g of compound Ⅰ;
[0044] (2) Hydrolysis reaction: 102.7 g of compound I obtained in step (1) was dissolved in 1800 g of aqueous sodium hydroxide solution with a mass concentration of 2.5%, to obtain a sodium hydroxide solution of compound II;
[0045] (3) Hydrogenation reaction: Add 1.5 g of hydrogenation catalyst platinum carbon to the sodium hydroxide solution of compound II obtained in step (2), first pass nitrogen protection into the reaction vessel, and then at a temperature of 50 ° C and a pressure of 2 MPa, Pass hydrogen into the sodium hydroxide solution of compound II, carry out the hydrogenation reaction for 5 hours, filter and recover the hydrogenatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com