Preparation method of polyfurandioctyl phthalate glycol ester
A technology of polyethylene furandicarboxylate and ethylene furandicarboxylate, which is applied in the field of bio-based polyester synthesis, can solve problems such as high molecular weight and color, and achieve improved product color, increased molecular weight, The effect of excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Add dimethyl furandicarboxylate (0.1mol, 18.4g), ethylene glycol (0.25mol, 15.5g) and 1,8-diazabicycloundec-7-ene (0.1mmol, 20.7 mg), reacted at 170°C for 1h, 180°C for 1h, and 190°C for 2 hours to obtain the transesterification product;
[0042] (2) Under the conditions of 250° C. and 150 Pa, the melt polycondensation reaction was carried out for 5 hours to obtain polyethylene furandicarboxylate PEF1.
[0043] After testing, the intrinsic viscosity of PEF1 is 0.83dL / g; the absorbance at 400nm is 0.05.
Embodiment 2
[0045] (1) Add dimethyl furandicarboxylate (0.1mol, 18.4g), ethylene glycol (0.25mol, 15.5g) and 1,8-diazabicycloundec-7-ene to coordinate the supported catalyst ( 0.1mmol, 16.8mg), reacted at 170°C for 1h, 180°C for 1h, and 190°C for 1 hour to obtain the transesterified product;
[0046] (2) Under the conditions of 240° C. and 150 Pa, the melt polycondensation reaction was carried out for 3 hours to obtain polyethylene furandicarboxylate PEF2.
[0047] After testing, the intrinsic viscosity of PEF2 is 0.96dL / g; the absorbance at 400nm is 0.03.
[0048] The preparation of the 1,8-diazabicycloundec-7-ene coordination supported catalyst comprises the following steps:
[0049] (i) Mix 10ml of n-butyl titanate in 100ml of hydrochloric acid with a concentration of 1mol / L, stir for 5h to obtain TiO 2 Sol;
[0050] (ii) In the above TiO 2 Add 4.5g talcum powder and 10g 1,8-diazabicycloundec-7-ene to the sol, stir for 3 hours, filter and centrifuge to obtain a white precipitate, w...
Embodiment 3
[0053] (1) Add dimethyl furandicarboxylate (0.1mol, 18.4g), ethylene glycol (0.25mol, 15.5g) and polyvinylpyrrolidone with a supported catalyst (0.1mmol, 20.9mg), react at 170°C for 1h, React at 180°C for 1 hour, and react at 190°C for 0.5 hour to obtain the transesterified product;
[0054] (2) Under the conditions of 250° C. and 200 Pa, the melt polycondensation reaction was carried out for 2 hours to obtain polyethylene furandicarboxylate PEF4.
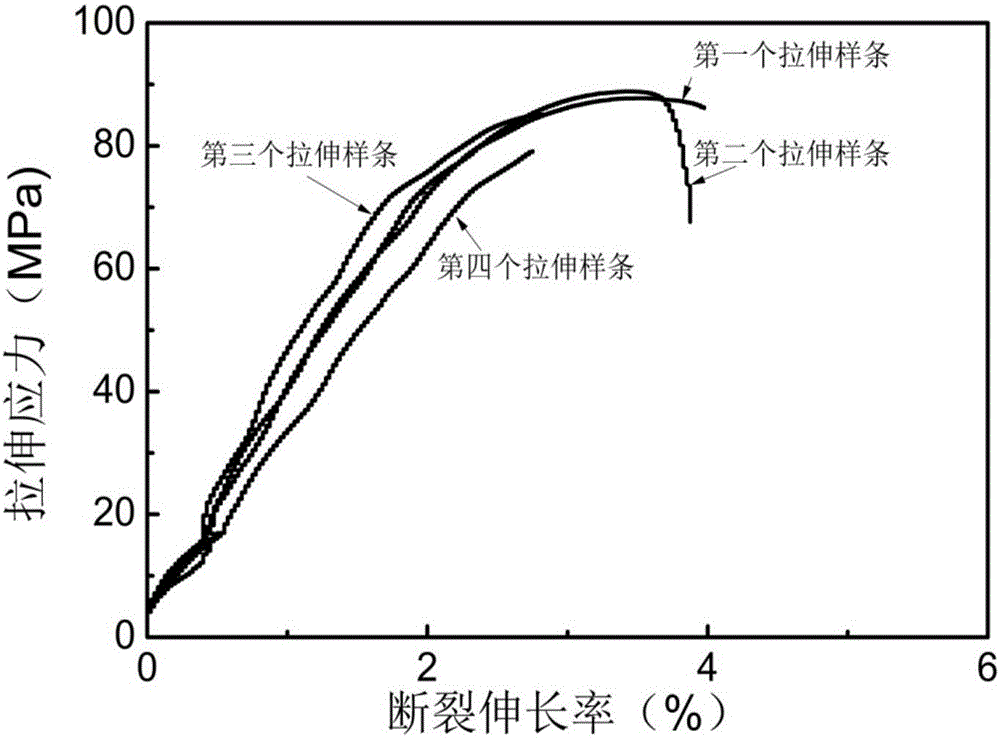
[0055] After testing, the intrinsic viscosity of PEF4 is 1.35dL / g; the absorbance at 400nm is 0.02; the mechanical properties of PEF4 are as follows figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com