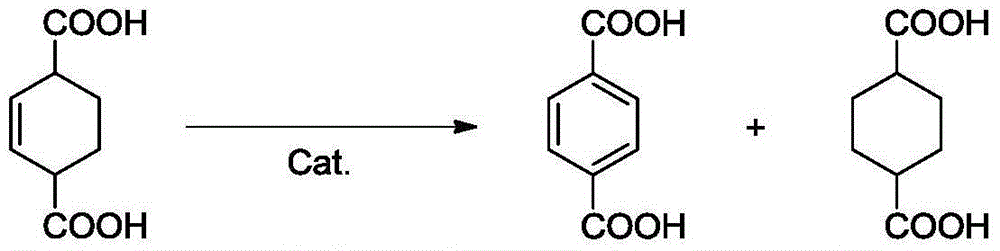
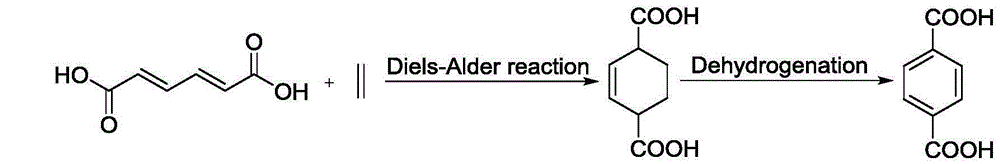Preparation method of terephthalic acid and diester thereof
A technology of terephthalic acid and dicarboxylic acid, applied in the field of chemical engineering or polymer science, can solve the problems of not complying with atomic economy, without bypassing PX, etc., to reduce dependence on petroleum resources, mild conditions, and atomic economy. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
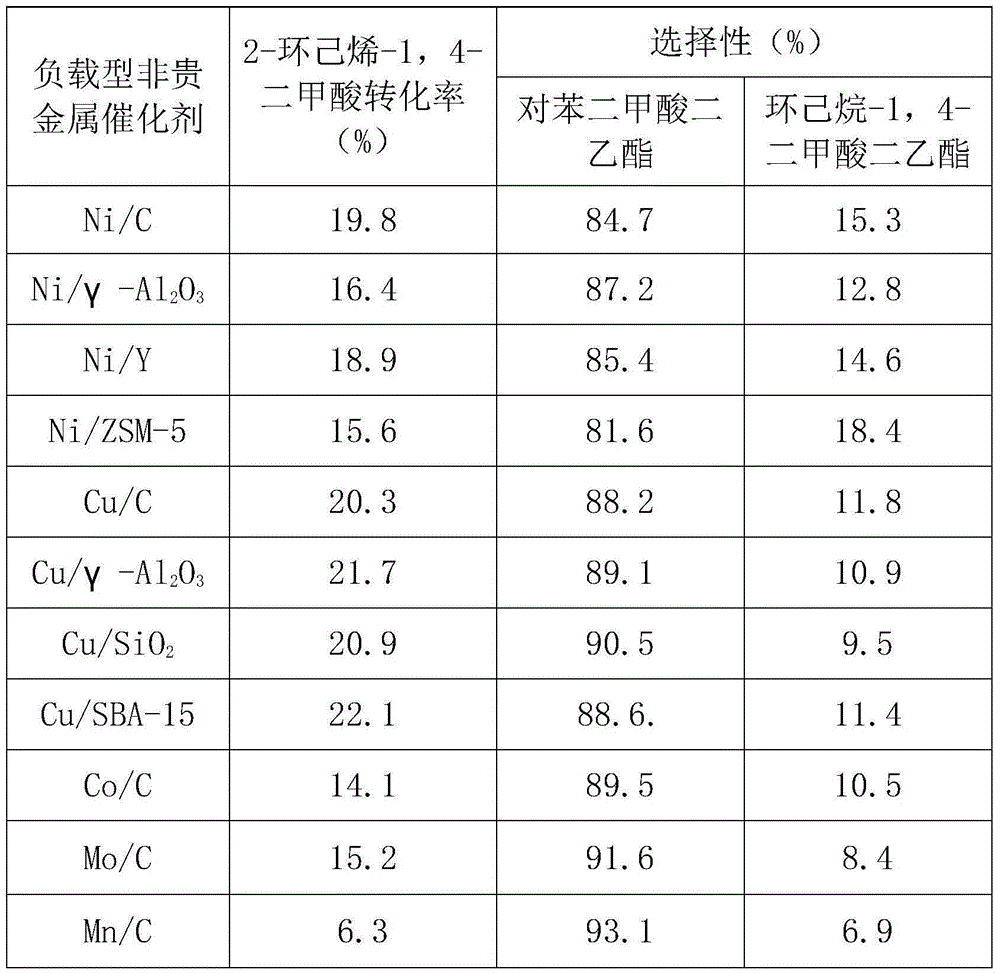
[0023] Example 1 Performance evaluation of supported non-precious metal catalysts for dehydrogenation of 2-cyclohexene-1,4-dicarboxylic acid
[0024] In this example, the performance of the supported non-noble metal catalyst in catalyzing the dehydrogenation reaction of 2-cyclohexene-1,4-dicarboxylic acid was studied.
[0025] In a high-pressure reactor with a polytetrafluoroethylene liner, add 1.58g of 2-cyclohexene-1,4-dicarboxylic acid and 15.8g of solvent ethanol, the mass of 2-cyclohexene-1,4-dicarboxylic acid and ethanol The ratio is 1:10, the molar ratio of the metal active component in the catalyst used to 2-cyclohexene-1,4-dicarboxylic acid is 1%, after stirring and mixing at room temperature, under nitrogen atmosphere, use electric heating Raise the temperature to 120° C., and react for 4 hours under magnetic stirring at 1000 rpm. Stop stirring, cool the reaction kettle to room temperature with ice water, take an appropriate amount of reaction solution to carry out ...
Embodiment 2
[0028] Example 2 Performance evaluation of supported noble metal catalysts for dehydrogenation of 2-cyclohexene-1,4-dicarboxylic acid
[0029] In this example, the performance of the supported noble metal catalyst in catalyzing the dehydrogenation reaction of 2-cyclohexene-1,4-dicarboxylic acid was studied.
[0030] In an autoclave with a Teflon liner, add 1.58g of 2-cyclohexene-1,4-dicarboxylic acid and 15.8g of solvent ethanol, 2-cyclohexene-1,4-dicarboxylic acid and ethanol The mass ratio is 1:10, the molar ratio of the metal active component of the catalyst used to 2-cyclohexene-1,4-dicarboxylic acid is 1%, after stirring and mixing at room temperature, under nitrogen atmosphere, use electric heating Raise the temperature to 120° C., and react for 4 hours under magnetic stirring at 1000 rpm. Stop stirring, cool the reaction kettle to room temperature with ice water, take an appropriate amount of the reaction solution to qualitatively analyze the product by gas chromatograph...
Embodiment 3
[0034] The influence of embodiment 3 solvent on 2-cyclohexene-1,4-dicarboxylic acid dehydrogenation reaction
[0035] In this example, the influence of different polar and non-polar solvents on the dehydrogenation reaction of 2-cyclohexene-1,4-dicarboxylic acid was studied.
[0036] Add the 2-cyclohexene-1,4-dicarboxylic acid reaction solution with a mass fraction of 10% into a high-pressure reactor with a polytetrafluoroethylene liner, and the reaction solution is composed of 2-cyclohexene-1,4-dicarboxylic acid Formic acid and the solvent composition described in Table 3, add the Pd / C catalyst that the metal active component is 2-cyclohexene-1,4-dicarboxylic acid molar weight 1%, after stirring and mixing at room temperature, under nitrogen atmosphere , using electric heating to raise the temperature to 120° C., and reacted for 4 hours under 1000 rpm magnetic stirring. Adopt the method of embodiment 1 to carry out qualitative and quantitative analysis to product. Table 3 sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com