Olive bitter glycoside suppository
A technology of oleuropein suppository and oleuropein, which is applied in the directions of suppository delivery, anti-inflammatory agent, non-central analgesic, etc., can solve the problems of no oleuropein suppository, bacterial resistance, difficult to control inflammation, etc. Good lubricating effect, comfortable medication, prolonged action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Immediate Release Oleuropein Suppository
[0019] (1) Get carbomer 0.3g, add water 29.7g, leave standstill 4h, make the carbomer solution that concentration is 1%, namely hydrogel;
[0020] (2) Take oleuropein, grind it finely, weigh 3g after passing through a 100-mesh sieve, add 3g of oleuropein to the hydrogel several times, and stir evenly;
[0021] (3) Weigh 198g of PEG4000, heat and melt it at 80°C, and then keep it at 60°C;
[0022] (4) Weigh 22 g of the hydrogel mixed with oleuropein prepared in step (2), add it to the melted PEG4000, heat and stir for 20 minutes, and mix it evenly;
[0023] (5) Pour the matrix containing oleuropein obtained in step (4) into the suppository mold while it is hot, and make 100 quick-release oleuropein suppositories, each weighing 2.2 g, each containing 20 mg of oleuropein, It contains 2.18g of matrix, in which PEG4000 accounts for 90.83%, and hydrogel accounts for 9.17%.
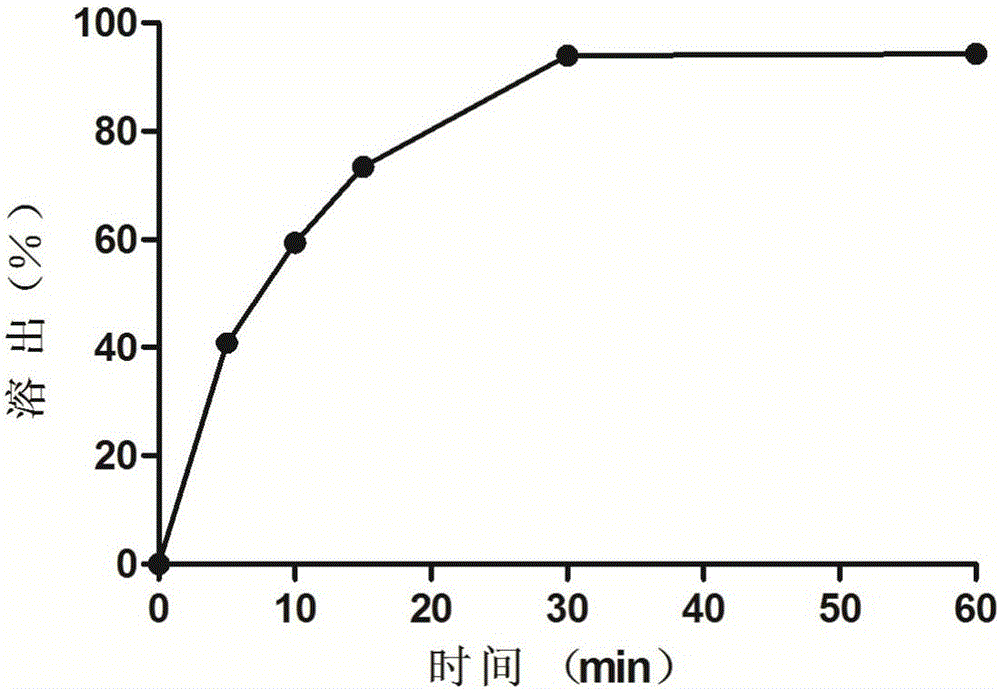
[0024] Get 6 prepared oleuropein suppositories...
Embodiment 2
[0025] Example 2 Immediate Release Oleuropein Suppository
[0026] (1) Take 109g of PEG400, add 2g of oleuropein, ultrasonically or slightly heat to dissolve the oleuropein;
[0027] (2) Weigh 99g of PEG4000, heat and melt it at 80°C, and then keep it at 60°C;
[0028] (3) Add the oleuropein-containing PEG400 obtained in step (1) into the melted PEG4000 several times, stir while adding, and continue stirring for 30 minutes after adding;
[0029] (4) The matrix containing oleuropein obtained in step (3) was poured into the suppository mold while it was hot, and 100 quick-release oleuropein suppositories were obtained, each weighing 2.1 g, each containing 20 mg of oleuropein, It contains 2.08g of matrix, in which PEG4000 accounts for 47.60%, and PEG400 accounts for 52.4%.
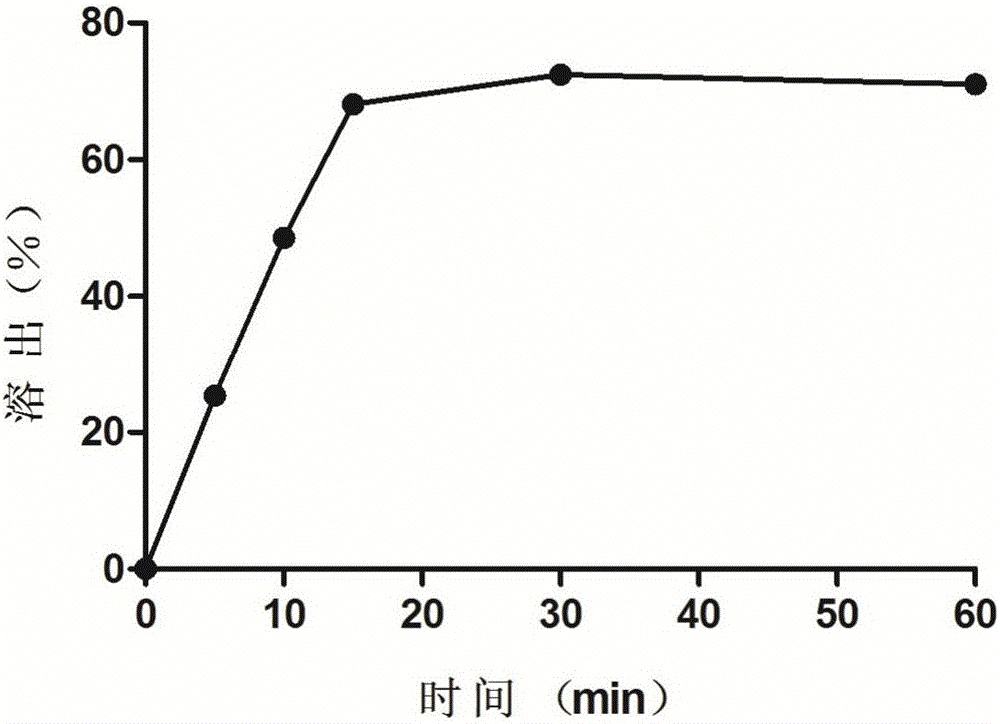
[0030] Get 6 prepared oleuropein suppositories to measure the hardness, the average hardness is 1.70 ± 0.22kg; according to the second method of the dissolution and release determination method of the 2015 ...
Embodiment 3
[0031] Example 3 Oleuropein Suppository with Moderate Release Rate
[0032] (1) Get 0.3 g of sodium carboxymethyl cellulose, add 29.7 g of water, let it stand for 4 hours, and obtain a sodium carboxymethyl cellulose solution with a concentration of 1%, namely hydrogel;
[0033] (2) Take oleuropein, grind it finely, weigh 3g after passing through a 100-mesh sieve, add 3g of oleuropein to the hydrogel several times, and stir evenly;
[0034] (3) Weigh 176g of PEG4000 and 22g of glyceryl monostearate, heat and melt them at 80°C, and then keep them warm at 60°C;
[0035] (4) Weigh 22 g of the hydrogel mixed with oleuropein prepared in step (2), add it to molten PEG4000 and glyceryl monostearate, heat and stir for 20 minutes, and mix it evenly;
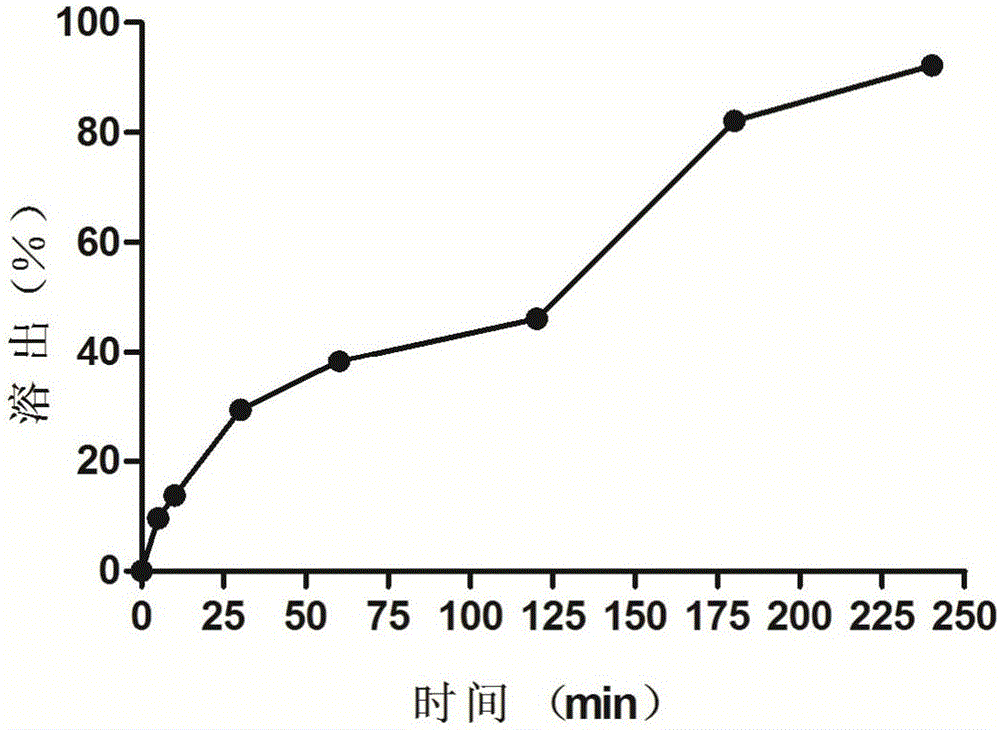
[0036] (5) Pour the oleuropein-containing matrix obtained in step (4) into the suppository mold while it is hot to prepare 100 medium-release oleuropein suppositories, each weighing 2.2g, and each containing oleuropein 20mg , containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com