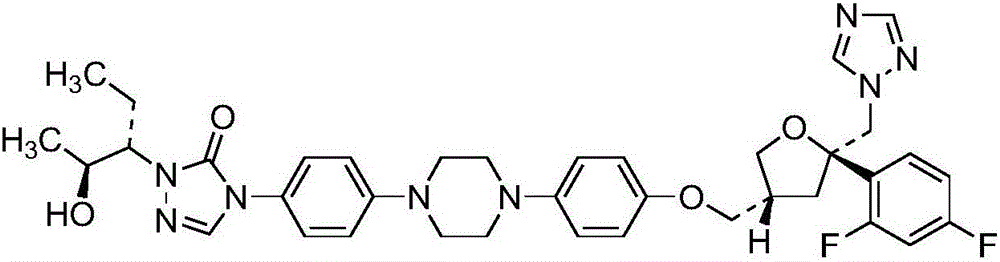
Antifungal drug posaconazole solid dispersion, preparation method and application
A technology for solid dispersions and antifungal drugs, which can be used in antifungal agents, pharmaceutical formulations, medical preparations with inactive ingredients, etc., and can solve the problems of increased content of related substances, low drug loading, and poor stability. The effect of promoting absorption, improving dissolution rate, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] making process:
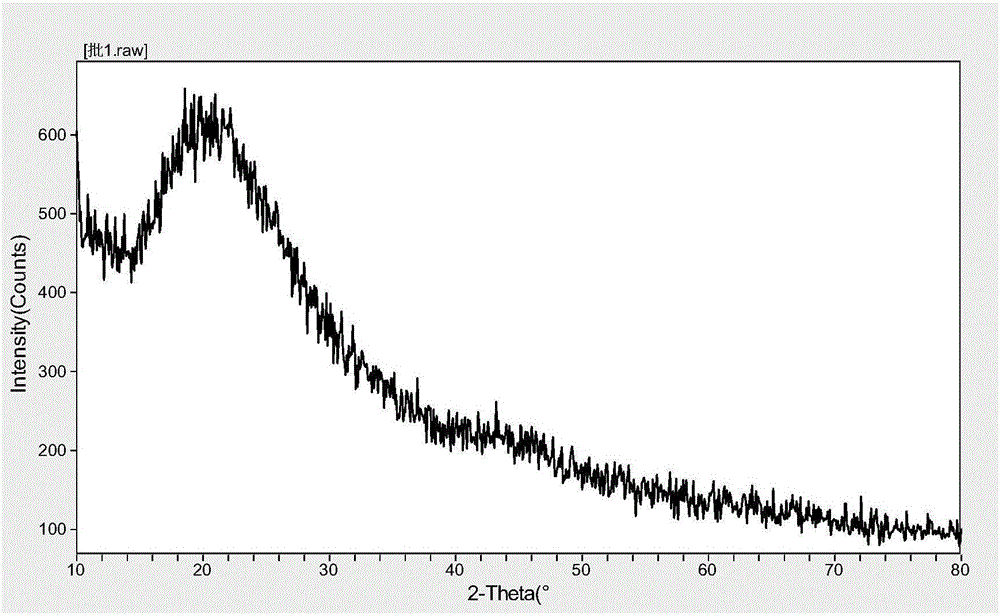
[0033] Weigh the prescribed amount of posaconazole and HPMC-AS MF into a flask, add a certain amount of 95% ethanol, dissolve in a water bath at 65°C, and then remove it by rotary evaporation at a vacuum of -0.03~-0.07M Pa at 75-80°C. part of ethanol, and then adjust the vacuum degree to be above -0.1M Pa to remove ethanol, take out the content and dry it in an oven at 40°C overnight to prepare posaconazole solid dispersion (its X-ray diffraction pattern is shown in the attached figure 1 ). Pulverize the posaconazole solid dispersion, pass through a 80-100 mesh sieve, add the prescribed amount of microcrystalline cellulose PH102, hydroxypropyl cellulose-EXF, and croscarmellose sodium, pass through a 80 mesh sieve and mix, and then Add the prescribed amount of MS, mix for 3 to 5 minutes, and use a rotary tablet press to directly compress the tablet.
Embodiment 2
[0035]
[0036] All the other processes are the same as in Example 1.
Embodiment 3
[0038]
[0039]
[0040] All the other processes are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com