Compound salt and crystal form or amorphous material and preparation methods thereof, pharmaceutical composition containing compound salt and crystal form or amorphous material and application
A crystal form and drug technology, applied in medical preparations containing active ingredients, organic chemistry methods, organic chemistry and other directions, can solve the problem of solution becoming turbid, achieve good aqueous solution stability, avoid unstable absorption, and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0135] According to the compound (I) seed crystal preparation method disclosed in the patent document WO2009020828A1, BMS-790052 free base was prepared as a starting material, and the specific operations were as follows:
[0136] 60.0g (105mmol, 1 equivalent) 4,4'-bis(2-((S)-pyrrolidinyl-2-yl)-1H-imidazol-5-yl) biphenyl, 38.7g (221mmol, 1 equivalent ) N-(methoxycarbonyl)-L-valine, 44.5g (232mmol, 2.2 equivalents) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 2.89g ( Add 21.4mmol, 0.2eq) of 1-hydroxybenzotriazole to 300mL of acetonitrile, stir to disperse, add 73.3mL (420.3mmol, 4eq) of diisopropylethylamine, and stir at 24-30°C for about 18 hours. Add 60 mL of water and heat to 50°C for about 5 hours. After cooling to room temperature, 320 mL of ethyl acetate and 300 mL of water were added, and the separated organic layer was washed with 300 mL of 10 wt % sodium bicarbonate aqueous solution, 300 mL of water and 200 mL of 10 wt % sodium chloride aqueous solution...
Embodiment 1
[0147] Example 1 Preparation of BMS-790052 di-p-toluenesulfonate
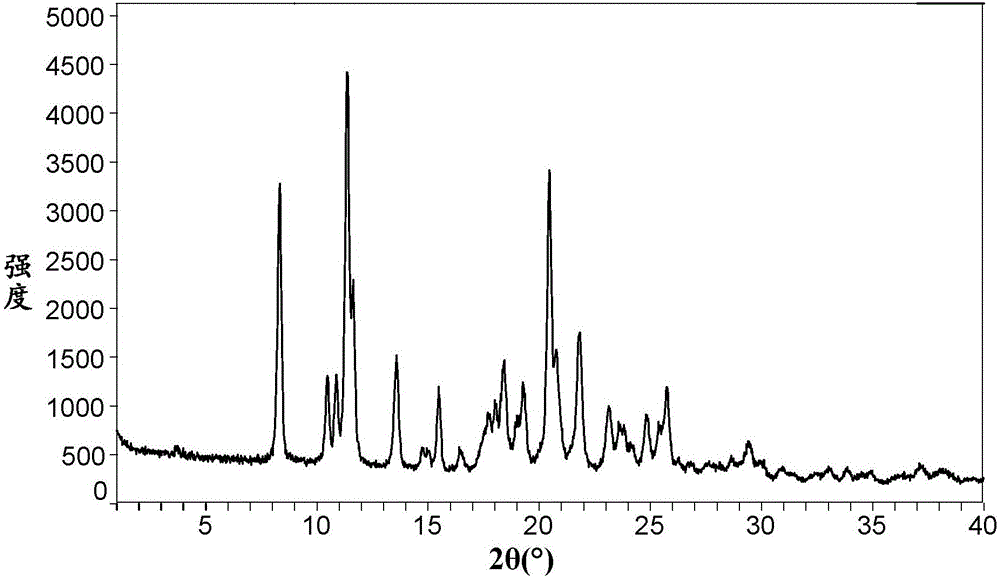
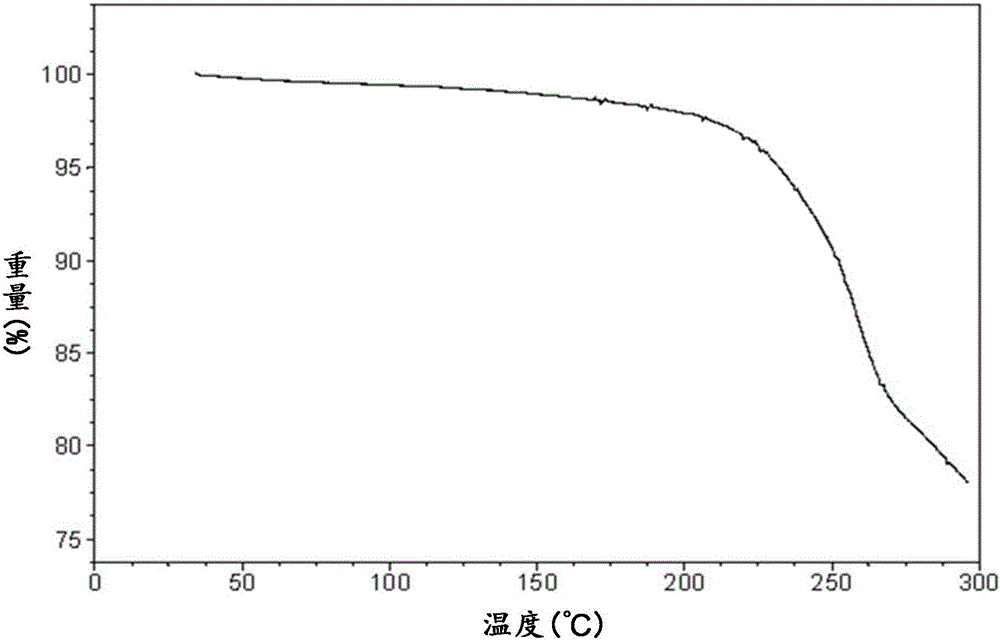
[0148] At room temperature, take 500 mg of the BMS-790052 free base prepared in Preparation Example 1, add 10 mL of acetone and dissolve it ultrasonically, add 256 mg of anhydrous p-toluenesulfonic acid solid to the acetone solution of the BMS-790052 free base, form a slurry and stir for 16 After 1 hour, it was filtered, and the filter cake was vacuum-dried at 40° C. for 16 hours to obtain 521 mg of BMS-790052 di-p-toluenesulfonate, with a yield of 71.1%.
[0149] As determined by HPLC, the actual content of BMS-790052 free base in BMS-790052 di-p-toluenesulfonate is 67.6%, and the theoretical content is 68.3%. The test results show that the free base of BMS-790052 and p-toluenesulfonic acid in the di-p-toluenesulfonate salt of BMS-790052 form a salt with a molar ratio of about 1:2.
Embodiment 2
[0150] Example 2 Preparation of BMS-790052 di-p-toluenesulfonate
[0151]At room temperature, take 50.0 mg of the BMS-790052 free base prepared in Preparation Example 1, add 2.0 mL of isopropanol and ultrasonically dissolve it, add 23.2 mg of anhydrous p-toluenesulfonic acid solid to the isopropanol solution of BMS-790052 free base, A slurry was formed and stirred. After stirring for 8 hours, it was filtered, and the filter cake was vacuum-dried at 40° C. for 16 hours to obtain 51.5 mg of BMS-790052 di-p-toluenesulfonate, with a yield of 70.3%.
[0152] The sample prepared in Example 2 has the same or similar HPLC detection results (not shown) as the sample in Example 1, indicating that the sample in Example 2 and the sample in Example 1 are the same substance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com