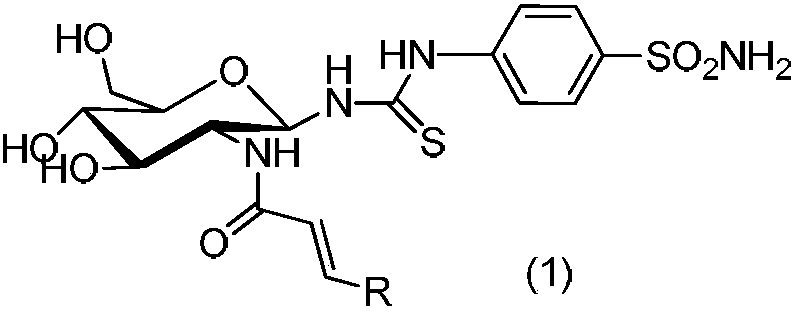
n-4-Benzenesulfonylamino-n'-1-deoxy-(2-deoxy-2-substituted amino)-β-d-glucopyranosylthiourea compound and its use
A kind of technology of glucopyranose amido group and benzenesulfonamide group, applied in N-4-benzenesulfonamide group-N'-1-deoxy-(2-deoxy-2-substituted amino)-β-D-pyran Glucosyl thiourea compounds and their application fields can solve the problem of difficulty in designing highly selective CAIX inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
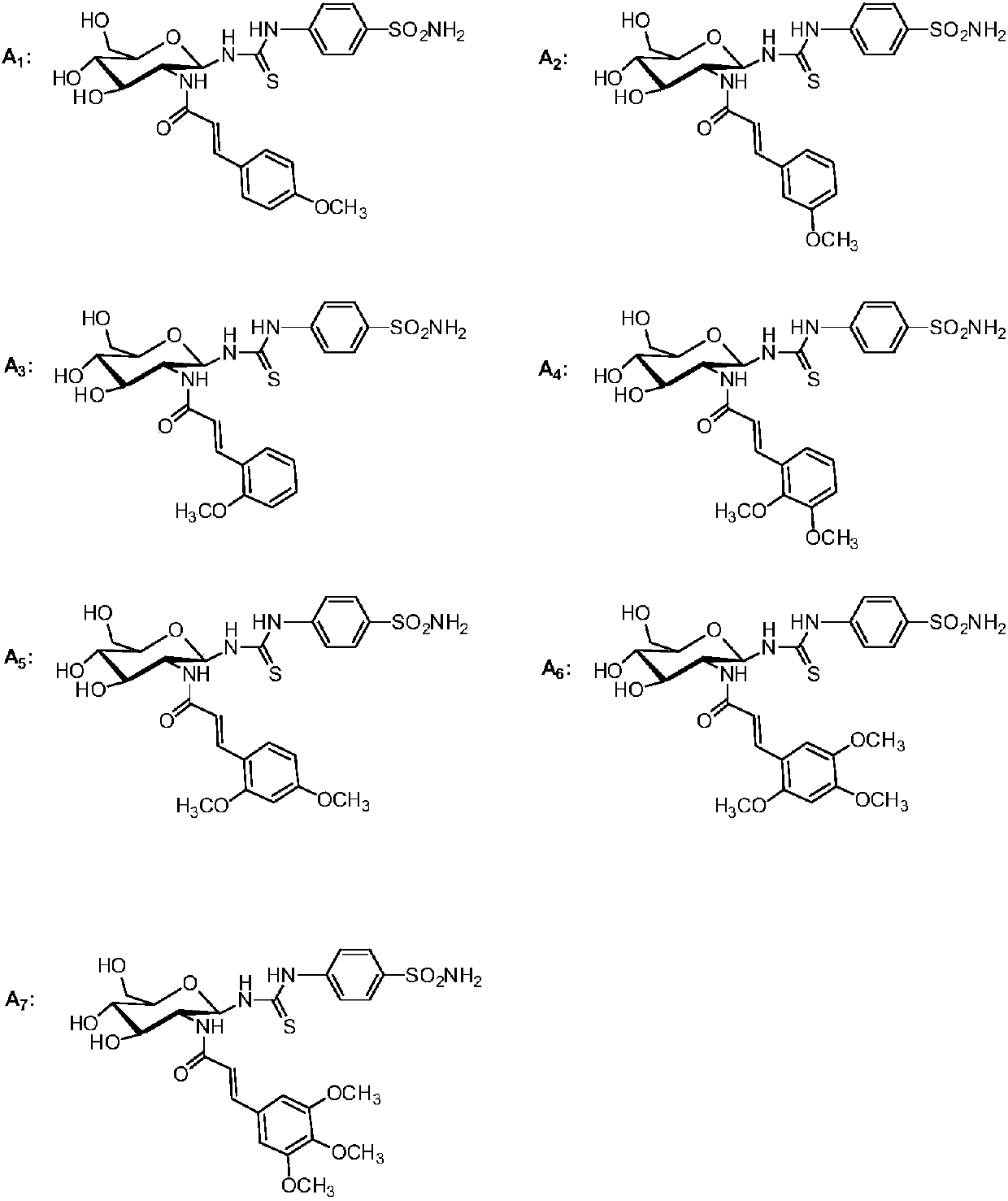
[0027] Example 1: N-4-benzenesulfonamido-N'-1-deoxy-[(2-deoxy-2-amino-N-trans-4'-methoxycinnamoyl)-β-D-pyran Glucosamido]thiourea (A1)
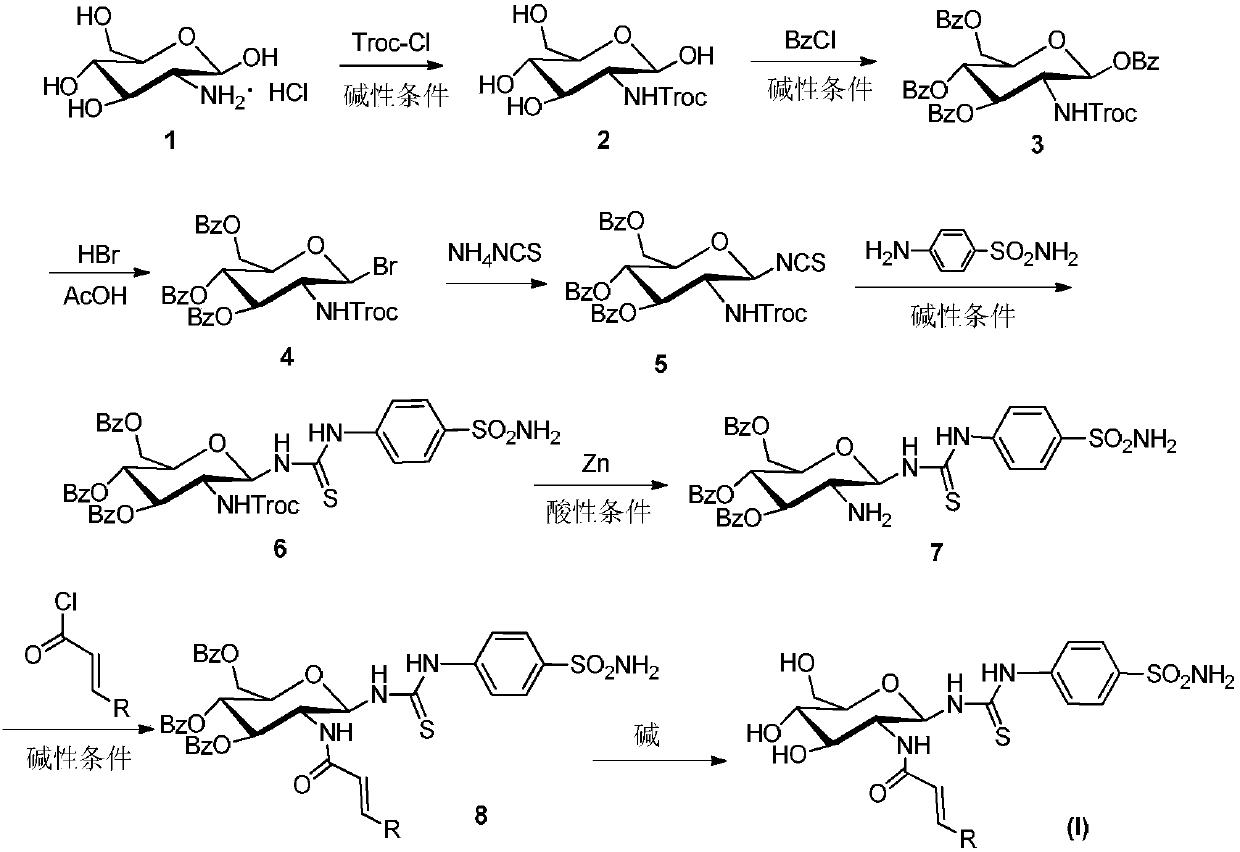
[0028] Dissolve glucosamine hydrochloride (15g, 69mmol) in 150mL of water, add sodium bicarbonate (17.39g, 207mmol) and trichloroethyl chloroformate (11.45mL, 83mmol), react for 5h, filter with suction, and dry to obtain white 20 g of solid, 90% yield.
[0029] The above product was suspended in pyridine (50 mL), and 70 mL of benzoyl chloride was added dropwise under ice-cooling, and reacted for 5 h. After the solution is clarified, add 200 mL of water and stir for 20 min, extract with dichloromethane 3 times, wash the organic layer 3 times with water, adjust the pH to 5 with dilute hydrochloric acid, adjust the pH to 7 with saturated sodium bicarbonate, wash 2 times with water, and dry over anhydrous sodium sulfate . After filtration, the solvent was removed under reduced pressure to obtain intermediate 3.
[0030] Dissolve the above cru...
Embodiment 2
[0037] Example 2: N-4-Benzenesulfonamido-N'-1-deoxy-[(2-deoxy-2-amino-N-trans-3'-methoxycinnamoyl)-β-D-pyran Glucosamido]thiourea (A2)
[0038] The preparation method of the compound in Example 2 was the same as in Example 1, except that m-methoxycinnamoyl chloride was used instead of p-methoxycinnamoyl chloride to obtain 57.4 mg of white solid with a yield of 89.8%.
[0039] Mp 170.9–172.7°C; 1 H NMR (600MHz, DMSO-d 6 )δ10.38(s,1H),8.33(d,J=6.6Hz,1H),8.03(s,1H),7.73(d,J=8.9Hz,2H),7.69(d,J=8.7Hz, 2H), 7.41(d, J=15.5Hz, 1H), 7.33(t, J=7.9Hz, 1H), 7.29(s, 2H), 7.14(d, J=7.4Hz, 1H), 7.12(s, 1H),6.97–6.94(m,1H),6.66(d,J=15.5Hz,1H),5.43(s,1H),5.10(s,2H),3.83(d,J=9.0Hz,1H), 3.78(s,3H),3.64(d,J=11.0Hz,1H),3.51(dd,J=11.8,4.4Hz,1H),3.44(dd,J=14.0,7.0Hz,2H),3.23(t , J=9.1Hz, 1H), 3.16 (d, J=8.8Hz, 1H); ESI-MS (m / z): 575.4[M+Na] + .
Embodiment 3
[0040] Example 3: N-4-Benzenesulfonamido-N'-1-deoxy-[(2-deoxy-2-amino-N-trans-2'-methoxycinnamoyl)-β-D-pyran Glucosamido]thiourea (A3)
[0041] The preparation method of the compound in Example 3 was the same as in Example 1, except that o-methoxycinnamoyl chloride was used instead of p-methoxycinnamoyl chloride to obtain 53.2 mg of a white solid with a yield of 83.3%.
[0042] Mp 172.9–174.5°C; 1 H NMR (600MHz, DMSO-d 6 )δ10.37(s,1H),8.31(s,1H),8.01(s,1H),7.72(d,J=14.0,8.8Hz,5H),7.52(d,J=7.3Hz,1H), 7.37(t, J=7.8Hz, 1H), 7.27(s, 2H), 7.07(d, J=8.3Hz, 1H), 6.99(t, J=7.5Hz, 1H), 6.70(d, J=15.7 Hz,1H),5.43(s,1H),5.07(d,J=20.3Hz,2H),4.52(s,1H),3.85(s,3H),3.65(s,1H),3.55–3.50(m ,1H),3.44(dd,J=7.0,5.1Hz,2H),3.24(dd,J=14.8,9.0Hz,1H),3.16(d,J=6.6Hz,1H); ESI-MS(m / z):575.5[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com