Exenatide detection kit
A detection kit, exenatide technology, applied in the field of biology and medical testing, can solve the problem of limiting the clinical application of GLP-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of Exenatide Detection Kit
[0022] 1) Preparation of conventional equipment and reagents:
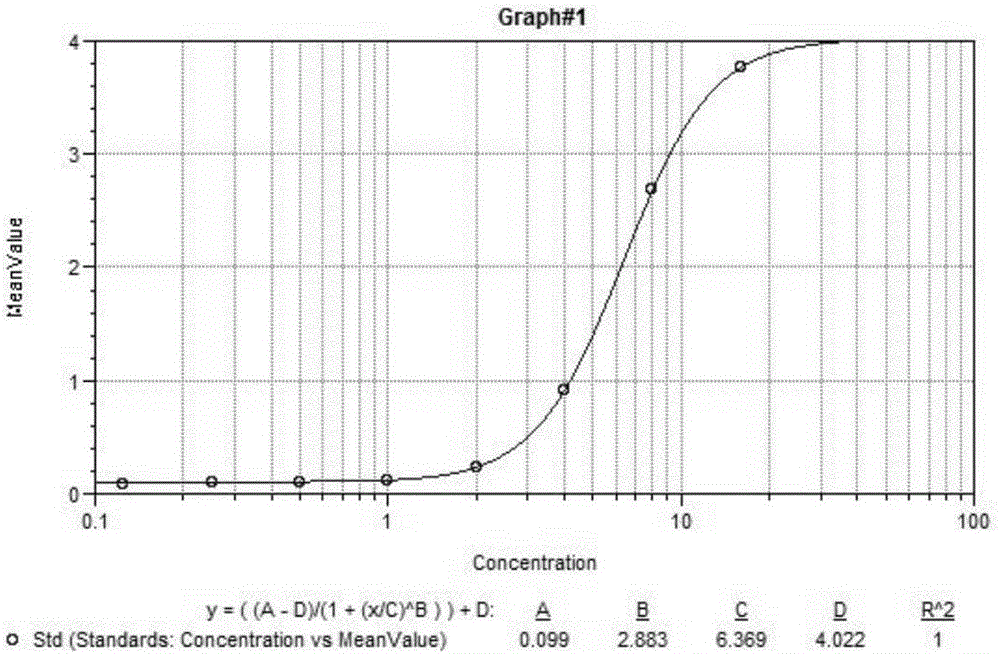
[0023] 96-well ELISA plate, exenatide positive serum, exenatide negative serum, washing solution, stop solution, horseradish peroxidase, and TMB color developing solution (which consists of color developing solution A, color developing solution B Composition), the coincidence rate of exenatide-positive sera and exenatide-negative sera was 100%.
[0024] 2) Preparation of exenatide monoclonal antibody:
[0025] a) In the first week, take 0.5ml of exenatide with a concentration of 1mg / ml and an equal volume of Freund's complete adjuvant to fully mix and emulsify, and immunize each female mouse respectively; 0.5ml of Exenatide with a concentration of 1mg / ml was fully mixed and emulsified with an equal volume of Freund's incomplete adjuvant, and each female mouse was immunized separately; blood was collected from the tail of the female mouse at the sixth week,...
Embodiment 2
[0035] Example 2: Use of Exenatide Detection Kit
[0036] 1) Add sample:
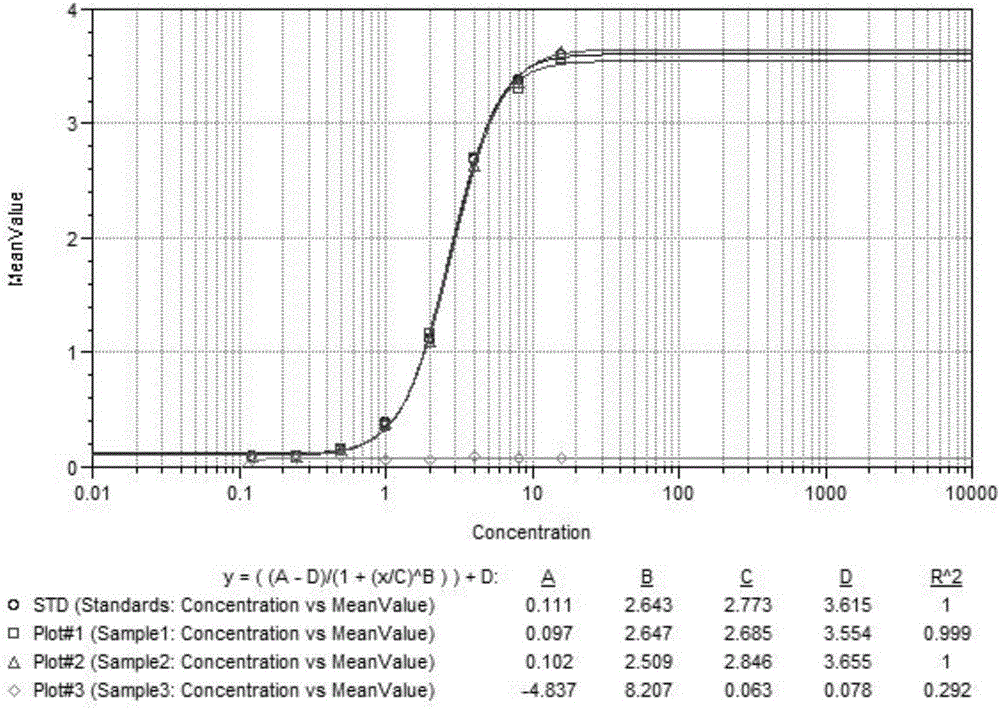
[0037] Exenatide monoclonal antibody 3D coated 7 The 96-well ELISA plate is divided into four groups of detection wells: positive control wells, negative control wells, test sample wells and blank wells. Exenatide positive serum is added to the positive control wells, and exenatide is added to the negative control wells. For negative serum, add the serum to be tested into the wells of the samples to be tested. The amount of the three samples added is 100 μl / well, then cover or cover the microplate, place the microplate at 37°C for 2 hours, discard Remove the liquid, wash with washing liquid, spin dry, and repeat the steps of washing and drying at least 3 times;
[0038] 2) Exenatide monoclonal antibody 5F labeled with horseradish peroxidase 3 :
[0039] Add 100 μl horseradish peroxidase-labeled exenatide monoclonal antibody 5F to each detection well 3 , put it at 37°C for 2 hours, discard the liqui...
Embodiment 3
[0044] Example 3: Detection of exenatide in animal serum:
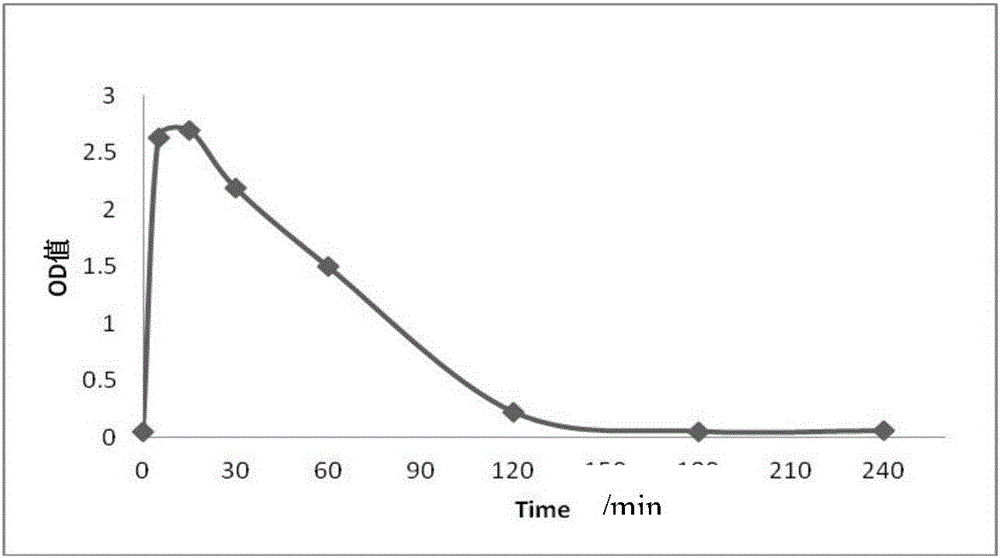
[0045] Experimental grade SD rats (about 6 weeks old) were selected, and exenatide injection (trade name: Byetta) was injected subcutaneously, and blood samples were collected at 0, 5, 15, 30, 60, 120, 180, and 240 min. Then adopt the kit and ELISA method that the disclosed method of the embodiment of the present invention makes to analyze the concentration of exenatide in the blood sample, and adopt microplate reader to measure, establish metabolic curve, the result is as follows figure 2 Shown: It can be seen from the figure that after subcutaneous injection of Byetta in SD rats, the exenatide in the blood reached the peak at about 15 minutes, and reached the lower limit of detection after 3 hours. By adopting the kit involved in the present invention, the concentration of exenatide in rat blood can be effectively detected, and a complete pharmacokinetic concentration curve can be established.
[0046] It should b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com