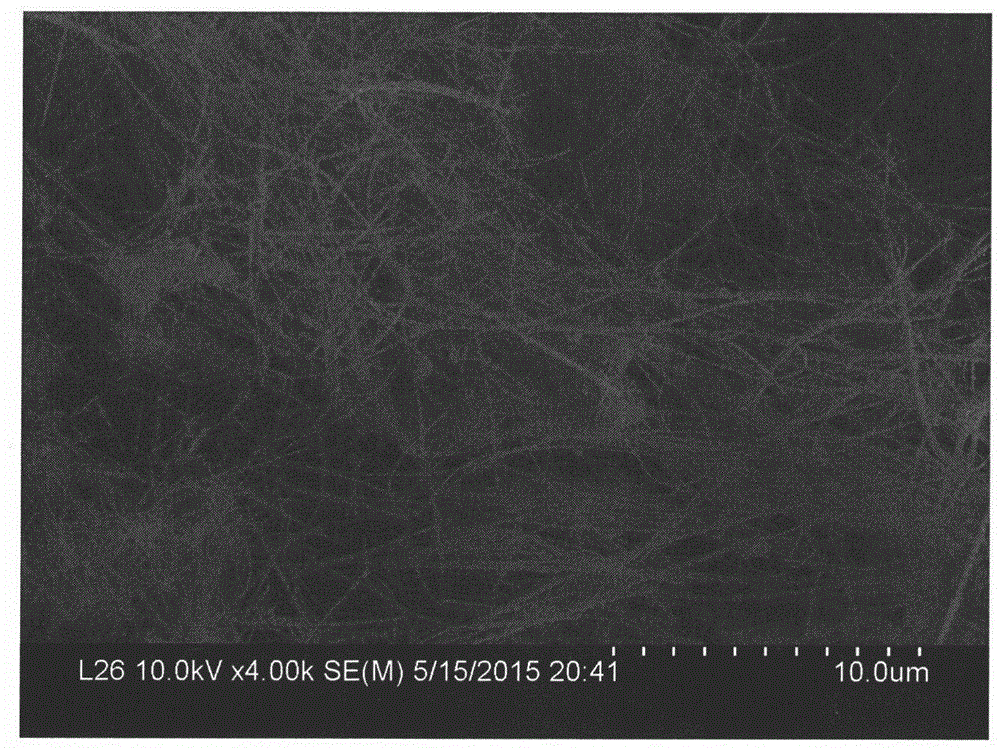
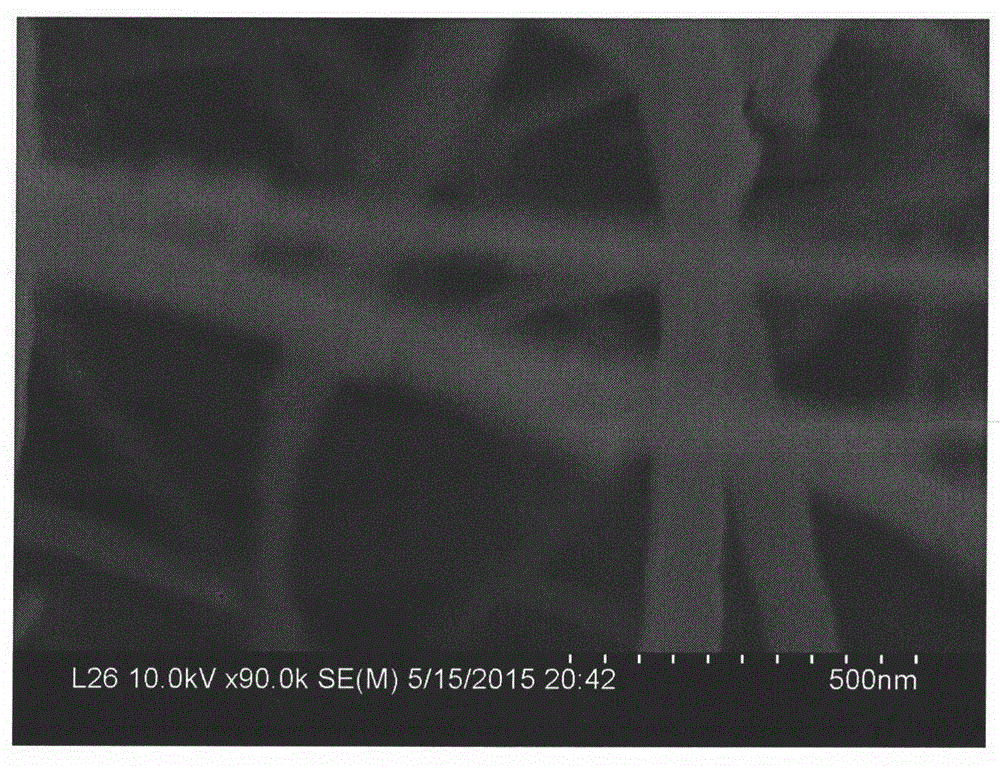
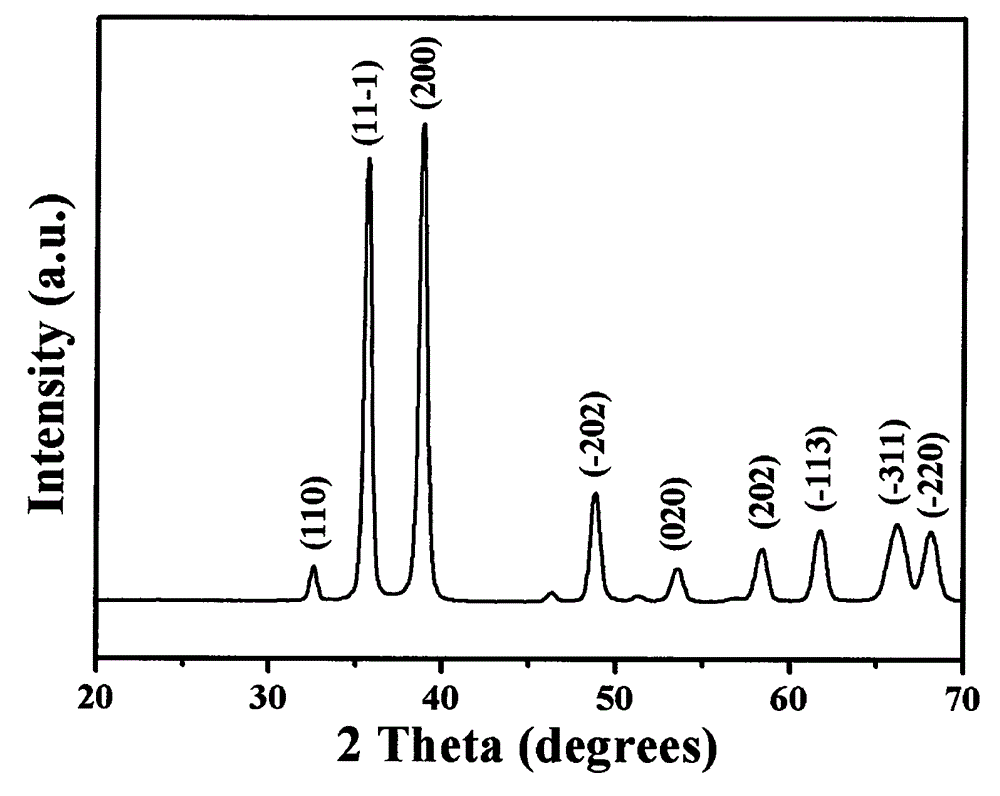
Preparation method of copper oxide nanowire
A technology of copper oxide nanowires and copper salts, applied in copper oxide/copper hydroxide, nanotechnology, nanotechnology, etc., to achieve the effects of cost reduction, high specific surface area, and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Dissolve 0.05g of polyvinylpyrrolidone (PVP) and 0.5g of urea in 4.5mL of deionized water / 2.5mL of glycerin to obtain solution A. Solution B was obtained by dissolving 2.5 mmol of copper chloride dihydrate (0.4262 g) in 2.5 mL of deionized water. Dissolve 0.5mmol ascorbic acid (0.0189g) in 2.5mL deionized water to obtain solution C;
[0016] (2) First drop solution B into solution A drop by drop while stirring at room temperature, after stirring evenly, drop solution C into the above mixed solution under strong stirring conditions, and pour the mixture into 50mL poly Hydrothermal reaction was carried out at 170°C for 16 hours in a tetrafluoroethylene autoclave.
[0017] (3) After the reaction, the obtained brick-red precipitate was taken out, then alternately centrifuged and washed with distilled water and absolute ethanol, then dried in a vacuum oven at 60°C, and then put into a muffle furnace and calcined at 500°C for 8h to obtain oxidation copper nanowires. Th...
Embodiment 2
[0019] (1) Dissolve 0.05g of polyvinylpyrrolidone (PVP) and 0.5g of urea in 4.5mL of deionized water / 2.5mL of glycerin to obtain solution A. Solution B was obtained by dissolving 2.5 mmol of copper sulfate pentahydrate (0.6242 g) in 2.5 mL of deionized water. Dissolve 0.5mmol ascorbic acid (0.0189g) in 2.5mL deionized water to obtain solution C;
[0020] (2) First drop solution B into solution A drop by drop while stirring at room temperature, after stirring evenly, drop solution C into the above mixed solution under strong stirring conditions, and pour the mixture into 50mL poly Hydrothermal reaction was carried out at 170°C for 16 hours in a tetrafluoroethylene autoclave.
[0021] (3) After the reaction, the obtained brick-red precipitate was taken out, then alternately centrifuged and washed with distilled water and absolute ethanol, then dried in a vacuum oven at 60°C, and then put into a muffle furnace and calcined at 500°C for 8h to obtain oxidation copper nanowires. ...
Embodiment 3
[0023] (1) Dissolve 0.05g of polyvinylpyrrolidone (PVP) and 0.5g of urea in 4.5mL of deionized water / 2.5mL of glycerin to obtain solution A. Solution B was obtained by dissolving 2.5 mmol of copper acetate monohydrate (0.4991 g) in 2.5 mL of deionized water. Dissolve 0.5mmol ascorbic acid (0.0189g) in 2.5mL deionized water to obtain solution C;
[0024] (2) First drop solution B into solution A drop by drop while stirring at room temperature, after stirring evenly, drop solution C into the above mixed solution under strong stirring conditions, and pour the mixture into 50mL poly Hydrothermal reaction was carried out at 170°C for 16 hours in a tetrafluoroethylene autoclave.
[0025] (3) After the reaction, the obtained brick-red precipitate was taken out, then alternately centrifuged and washed with distilled water and absolute ethanol, then dried in a vacuum oven at 60°C, and then put into a muffle furnace and calcined at 500°C for 8h to obtain oxidation copper nanowires.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com