Histone deacetylase inhibitor and preparation method and application thereof
A deacetylase and histone technology, applied in chemical instruments and methods, anti-inflammatory agents, drug combinations, etc., can solve the problems of cell necrosis, cell differentiation, cell apoptosis, etc., and achieve strong inhibitory effect and high selectivity. , active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
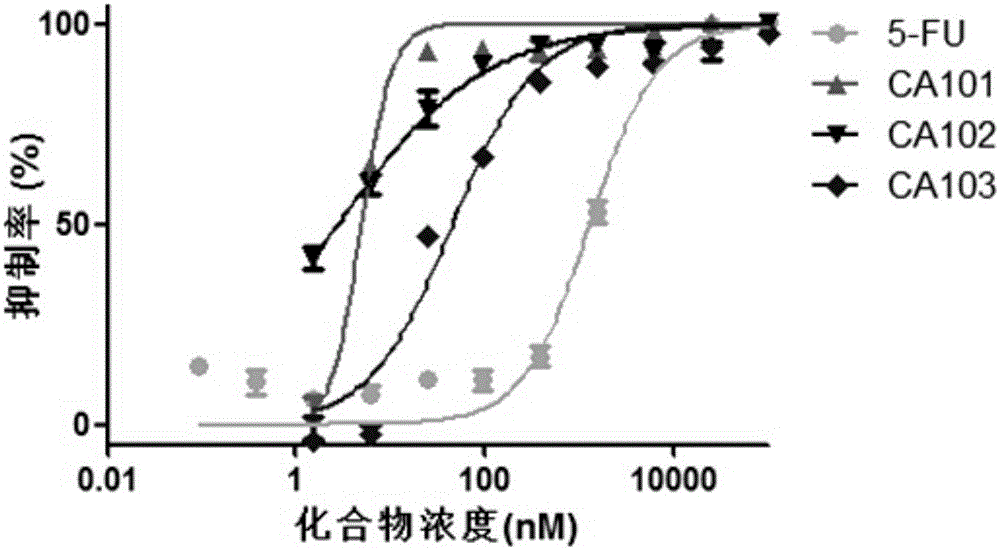
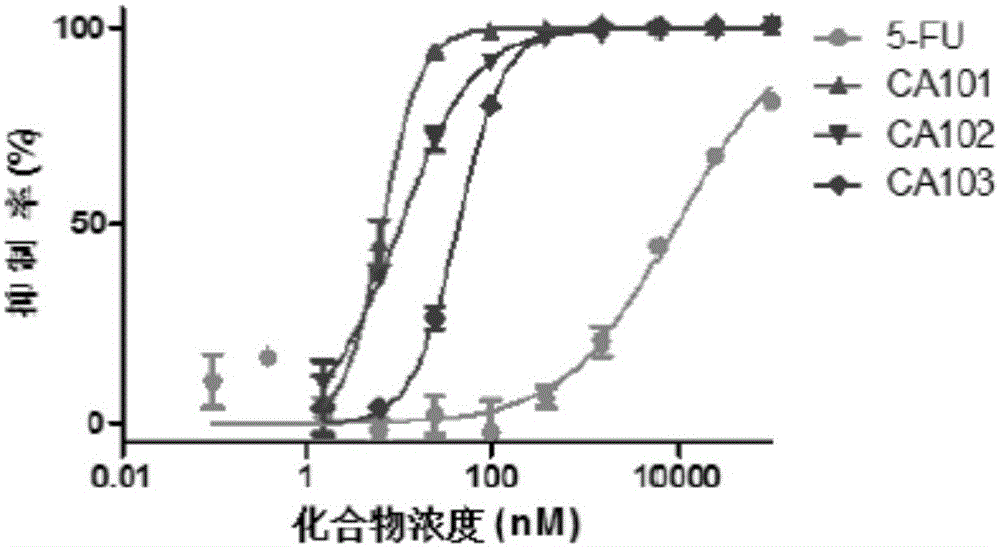
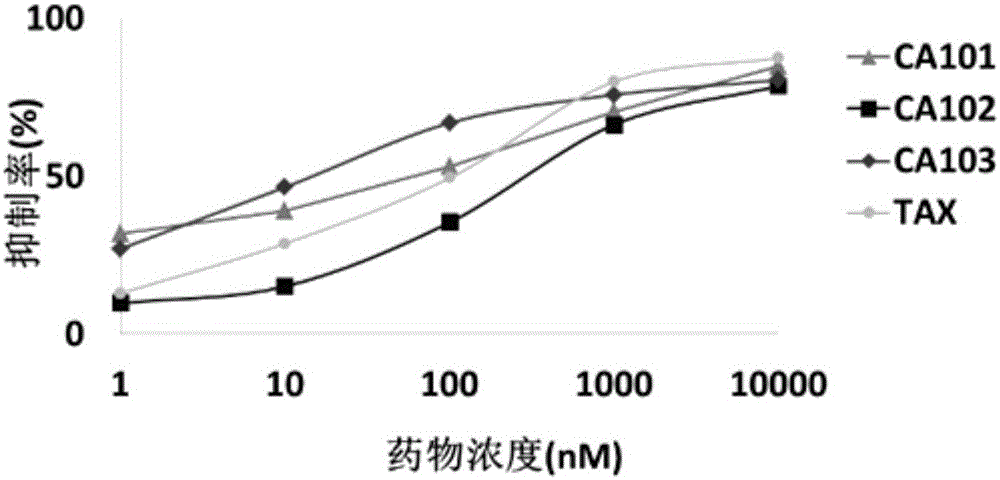
Image
Examples
Embodiment 1
[0063] Embodiment 1: The concrete preparation method of synthesizing CA101 comprises the following steps:
[0064] Step 1, prepare CA101-2:
[0065] The structural formula of CA101-2 is:
[0066] Weigh raw material CA101-1 (2.88g, 4.54mmol), the structural formula of CA101-1 is Place in a 50mL flask, add 25mL of redistilled dichloromethane, stir to dissolve all the solids, and then slowly add 5.76mL of trifluoroacetic acid dropwise. After the dropwise addition was completed, the reaction was stirred at room temperature for 2 hours, and the progress of the reaction was checked by spotting a TLC plate, and there was no raw material remaining. The reaction solution was spin-dried, then dissolved in toluene (5 mL×2) and spin-dried. Then, it was dried with a low-pressure oil pump for 1 hour to obtain a light brown transparent semi-solid, which was directly used in the next reaction without further purification.
[0067] Step 2, prepare CA101-4:
[0068] The structural formu...
Embodiment 2
[0088] Embodiment 2: the concrete preparation method of synthesizing CA102 comprises the following steps:
[0089] Step 1, prepare CA102-2:
[0090] The structural formula of CA102-2 is:
[0091] Get CA102-1 (2.4g, 8.2mmol), its structural formula is Dissolve it in 24mL of dichloromethane, then add 4.8mL of trifluoroacetic acid dropwise, and complete the addition within 10 minutes. After stirring at room temperature for 2 hours, it was spin-dried and banded twice with toluene (5 mL×2). After the oil pump is drained for 1 hour, it is directly cast to the next step.
[0092] Step 2 prepares CA102-3:
[0093] The structural formula of CA102-3 is:
[0094] Dissolve CA102-2 (1.57g, 8.2mmol) in 30mL of anhydrous N,N-dimethylformamide, at 0°C, add 1-ethyl-(3-dimethylaminopropyl)carbodi Imine hydrochloride (3.1g, 16.4mmol), 1-hydroxybenzotriazole (2.1g, 16.4mmol) and sodium bicarbonate (4.2g, 49.2mmol), then add N-Boc-alanine (1.87 g, 9.8 mmol), stirred at 0°C for 10 minut...
Embodiment 3
[0120] The concrete preparation method of synthetic CA103 comprises the following steps:
[0121] Dissolve CA102 (20 mg, 0.03 mmol) in 1 mL redistilled dichloromethane under nitrogen protection. Add Dess-Martin oxidizer (DMP) (34mg, 0.075mmol) under ice-bath at 0°C, stir at 0°C for 10 minutes, then at room temperature for 12 hours. After the reaction was monitored by TLC, cool down to 0°C, add 1 mL of dichloromethane to dilute, then add 1 mL of saturated sodium bicarbonate and 1 mL of 1M sodium thiosulfate successively, stir for 10 minutes and then separate the liquids, and use dichloromethane (3 mL× 3) Extract, combine the organic phases, wash with 3 mL of saturated brine, and dry over anhydrous sodium sulfate.
[0122] After spin-drying and passing through the column (DCM / MeOH=50 / 1), a colorless oil (CA103) was obtained, 15 mg, yield 75.4%.
[0123] CA103 NMR data:
[0124] 1 H NMR (400MHz, CDCl 3 )δ7.78(d, J=8.4Hz, 1H), 7.60(d, J=7.1Hz, 1H), 7.01(d, J=6.7Hz, 1H), 5.76–...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com