Intumescent flame retardant of borate derivative and preparation method thereof
A technology of intumescent flame retardants and derivatives, applied in the field of intumescent flame retardants, can solve problems such as toxicity and weak flame retardant performance, and achieve the effects of preventing dripping, preventing combustion, and stabilizing the carbon layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
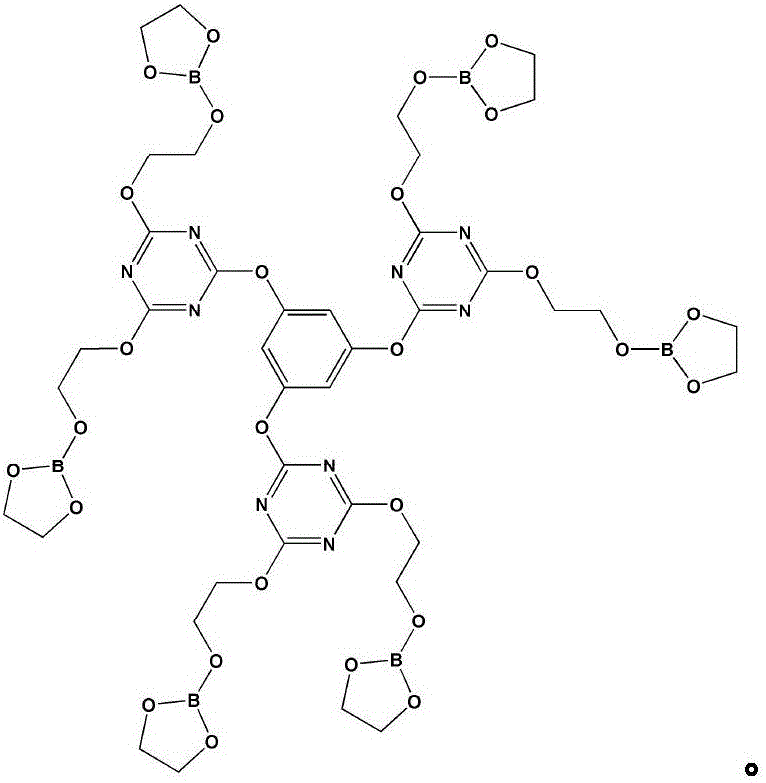
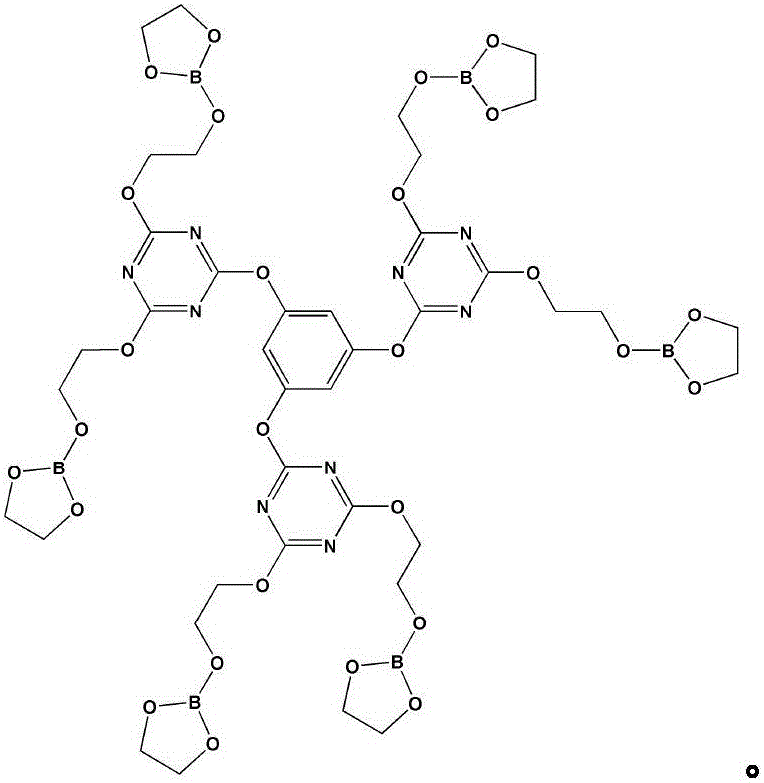
[0029] The first step: 2.58g phloroglucinol and 9.11gN, N-diisopropylethylamine are dissolved in 40ml tetrahydrofuran solution (mixture 1), 16.79g cyanuric chloride is dissolved in 80ml tetrahydrofuran solution, and Fully stir in an ice-water bath for 31 minutes (mixed solution 2), add mixed solution 1 dropwise to mixed solution 2, stir at 5°C for 3 hours, and filter;
[0030] The second step: use a rotary evaporator to remove tetrahydrofuran in the filtrate of the second step to obtain a white solid, use a mixture of petroleum ether and acetone (3:1 by volume) as a flushing solution, and separate with a chromatographic column to obtain an intermediate product 1;
[0031] Step 3: Add 13.5ml ethylene glycol and 30ml toluene solution into the three-necked flask, stir and gradually raise the temperature to 42°C, add 7.34g boric acid, continue to heat up to 88°C, stir for 47 minutes until the boric acid is completely dissolved; The moisture was no longer increased, and the reacti...
Embodiment 2
[0039] The first step: 5.23g phloroglucinol and 18.37g N,N-diisopropylethylamine are dissolved in 80ml tetrahydrofuran solution (mixture 1), 35.31g cyanuric chloride is dissolved in 80ml tetrahydrofuran solution, And fully stir in the ice-water bath for 31min (mixed solution 2), add the mixed solution 1 dropwise to the mixed solution 2, stir at 5°C for 3h, and filter;
[0040] The second step: use a rotary evaporator to remove tetrahydrofuran in the filtrate of the second step to obtain a white solid, use a mixture of petroleum ether and acetone (3:1 by volume) as a flushing solution, and separate with a chromatographic column to obtain an intermediate product 1;
[0041] Step 3: Add 27ml of ethylene glycol and 60ml of toluene solution into the three-necked flask, stir and gradually heat up to 42°C, add 14.89g of boric acid, continue to heat up to 88°C, and stir for 47 minutes until the boric acid is completely dissolved; Continue to react for 2.5 h without further increase; ...
Embodiment 3
[0045] The first step: 7.76g phloroglucinol and 27.29gN, N-diisopropylethylamine are dissolved in 120ml tetrahydrofuran solution (mixture 1), 49.63g cyanuric chloride is dissolved in 240ml tetrahydrofuran solution, and Fully stir in an ice-water bath for 37 minutes (mixed solution 2), add mixed solution 1 dropwise to mixed solution 2, stir at 3°C for 5 hours, and filter;
[0046] The second step: use a rotary evaporator to remove tetrahydrofuran in the filtrate of the second step to obtain a white solid, use a mixture of petroleum ether and acetone (3:1 by volume) as a flushing solution, and separate with a chromatographic column to obtain an intermediate product 1;
[0047] Step 3: Add 41ml of ethylene glycol and 90ml of toluene (as a water-carrying agent) solution into the three-necked flask, stir and gradually heat up to 47°C, add 22.67g of boric acid, continue to heat up to 70°C, and stir for 55 minutes until the boric acid is completely dissolved ; When the moisture in...
PUM
| Property | Measurement | Unit |
|---|---|---|
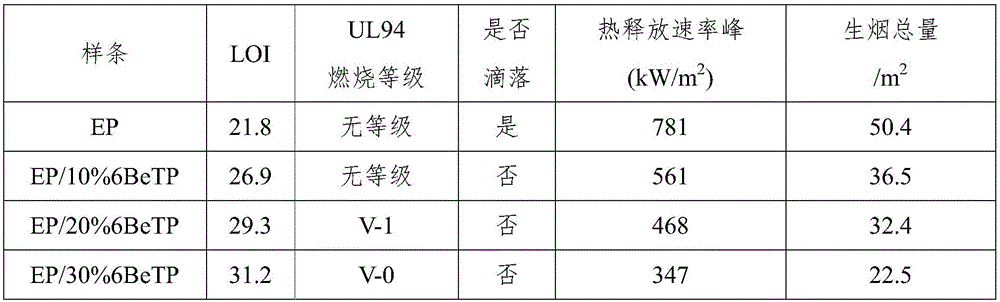
| heat release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com