A kind of industrial preparation method and application of recombinant interferon gamma
A technology of porcine interferon and purification method, which is applied in the direction of peptide preparation method, biochemical equipment and method, interferon, etc., can solve the problems of lowering the specific activity rate of recombinant protein, unqualified product quality, precipitation and other problems, and achieve easy scale-up and repeatable, reduced content, easy-to-amplify effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
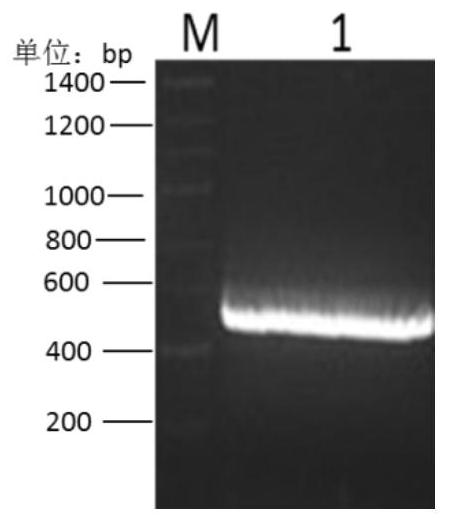
[0052] Example 1 Construction and expression identification of rpIFNγ Escherichia coli strain
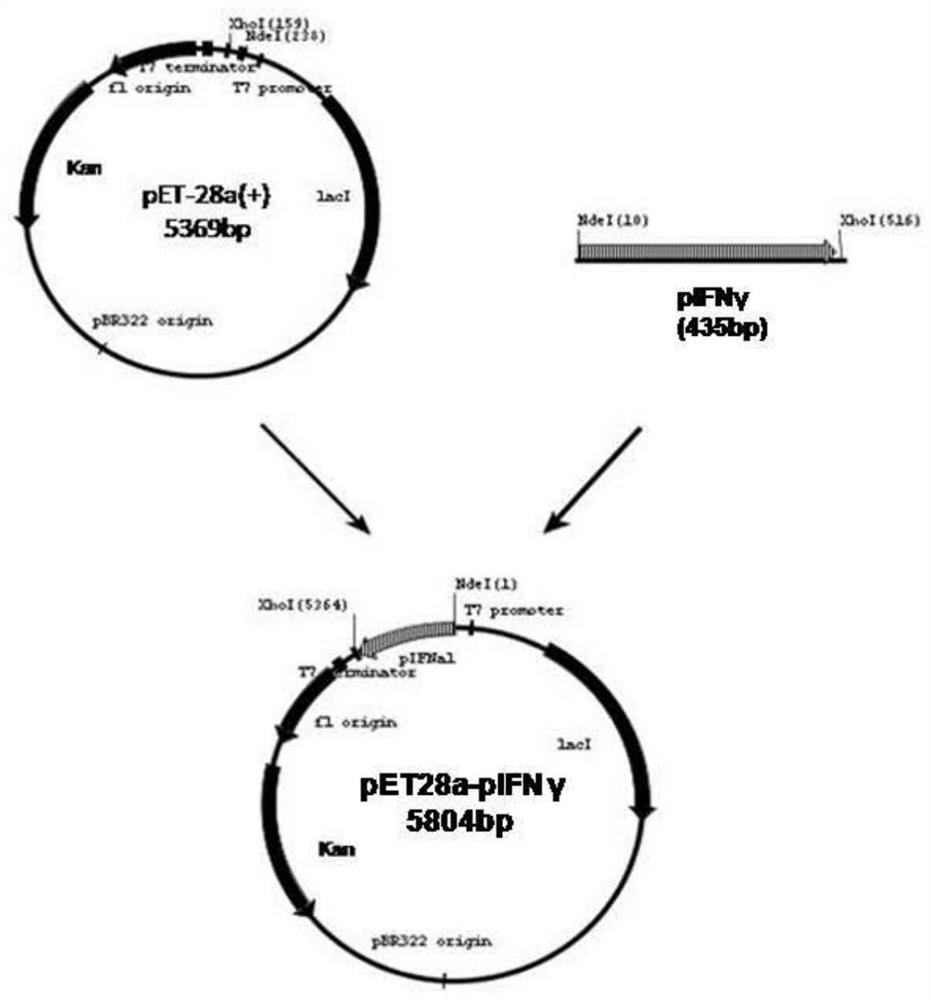
[0053] The inventor has previously completed codon optimization of the rpIFNγ gene for E. coli and mRNA free energy optimization, and inserted the optimized rpIFNγ gene into the pET21b vector to construct a recombinant expression plasmid, denoted as pET21b-rpIFNγ; the expression plasmid pET21b-rpIFNγ It was introduced into the expression host of E. coli BL21(DE3) (denoted as BL21(DE3)-pET21b-rpIFNγ) by means of molecular cloning to achieve high protein expression, as described in the patent (application number: 201210593705.X). To clone the rpIFNγ gene from the pET21b vector into pET28a, see the following steps:
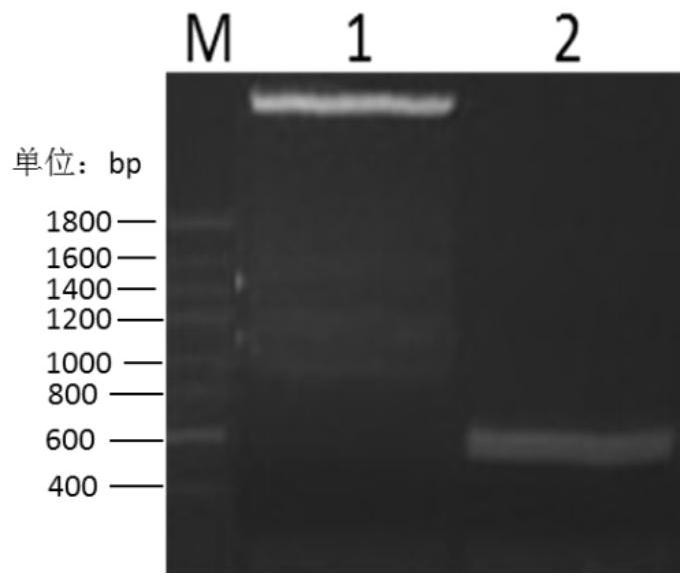
[0054] 1. Take glycerol tubes of BL21(DE3)-pET21b-rpIFNγ and DH5α-pET28a strains frozen in a -80°C refrigerator, inoculate them in LB liquid medium after thawing, and use the plasmid mini-extraction reagent after culturing overnight on a shaker at 37°C at 220 rpm The plas...
Embodiment 2
[0062] Example 2 High-density fermentation of rpIFNγ
[0063] This example mainly describes the high-density fermentation process of the BL21(DE3)-pET28a-rpIFNγ strain. In the fermentation process, glucose and glycerol with clear components are used as the carbon source for bacterial metabolism, and ammonia water (used for pH adjustment during the fermentation process) It is also used as a nitrogen source for cell metabolism) and diammonium hydrogen phosphate as a nitrogen source for cell metabolism; since no compound medium similar to yeast powder and peptone is used, the compound nitrogen source / carbon source is avoided. Therefore, the medium composition of different fermentation batches can be easily controlled, and because the cost of glucose and glycerol is low, the overall cost of the fermentation process is well controlled. Fermentation cell OD after fermentation 600 It can reach between 90-100, and the expression level of the target protein can reach more than 1.5g / L....
preparation example 1
[0064] Preparation example 1 concrete steps are as follows:
[0065] 1. Preparation of fermented seed liquid: inoculate the cryopreservation tube of the rpIFNγ E. coli strain constructed in Example 1 into LB solid medium containing 50 μg / mL kanamycin using the method of three-section line, and overnight at 37°C culture for activation;
[0066] 2. Pick a single colony with a plump shape and a moderate size from the solid medium and inoculate it into LB liquid medium containing 50 μg / mL kanamycin, and culture it on a shaker at 220 rpm at 37°C for 8 hours. This is the primary seed solution.
[0067] 3. Transfer the primary seed solution in step 2 to fresh LB liquid medium containing 50 μg / mL kanamycin according to the inoculum amount of 1%, and culture it on a shaker at 37°C and 220 rpm until OD600≈3-5 Between, this is the preparation of secondary seed solution.
[0068] 4. Composition of batch fermentation medium: citric acid monohydrate 1.5g / L, potassium dihydrogen phosphate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com