Naphtho-thioxanthene derivative as well as preparation method and application thereof
A technology for naphthothanthene and derivatives, which is applied in the field of naphthothanthene derivatives and their preparation, can solve the problems of underappreciated, low driving voltage luminous efficiency and the like, and achieves improved luminous efficiency and excellent electron transport capability. , the preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
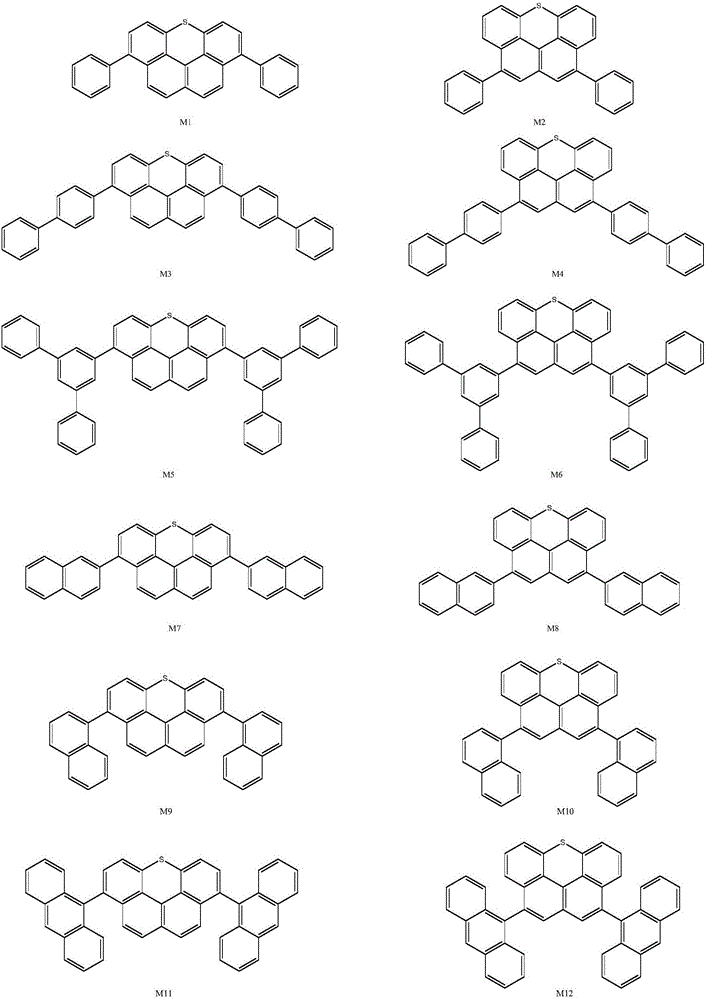
Image
Examples
preparation example Construction
[0041] The present invention also provides a preparation method of naphthothioxanthene derivatives, comprising:
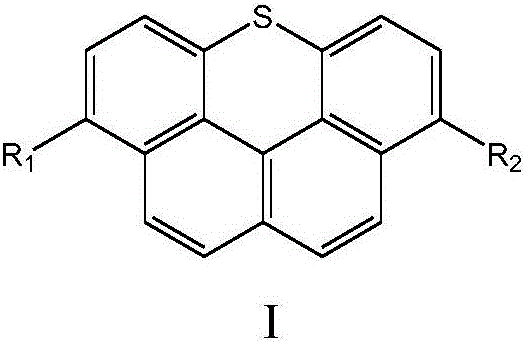
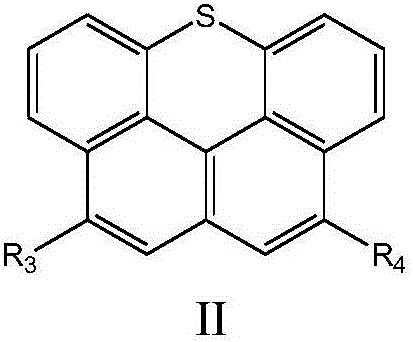
[0042] Under the protection of nitrogen, the compound represented by the intermediate formula A or the compound represented by the intermediate formula B is subjected to a coupling reaction to obtain a naphthothioxanthene derivative represented by formula I or formula II;
[0043]
[0044] The R 1 , R 2 , R 3 , R 4 The selection of the groups is the same as above, and will not be repeated here.
[0045] According to the intermediate A of the present invention, it is prepared according to the following method:
[0046] (1) Nitrating the thioxanthone shown in formula A-1 with nitric acid to obtain the disubstituted nitro compound shown in formula A-2;
[0047] (2) The compound shown in formula A-2 and carbon tetrabromide are subjected to a Corey-Fuchs dibromoalkenylation reaction in the presence of triphenylphosphine to obtain a dibromoalkene compound shown i...
Embodiment 1
[0064] Embodiment 1: the preparation of intermediate A
[0065] (1) Synthesis of Compound A-2: Add 150ml of fuming nitric acid into a 500ml reaction flask, cool to about 5°C in an ice-water bath, add 21.2g (0.1mol) of thioxanthone (Compound A-1) in batches under stirring ), control the reaction temperature not to exceed 10°C, keep the temperature of the reaction solution at 5°C after adding the reactant, and react for about 30min. The reactant was poured into ice water, stirred vigorously, and filtered with suction. The filter cake was washed with water, dried and recrystallized to obtain compound A-2 with a yield of 80%.
[0066] (2) Synthesis of Compound A-3: 22.6g of Compound A-2 (0.075mol) and 50g of carbon tetrabromide (0.15mol) were added to a 500ml dry reaction flask, and 250ml of Benzene was dried, stirred for 5 min, and 78.6 g of triphenylphosphine (0.3 mol) was added. Stir the reaction mixture at 150°C and react for 48 hours. After the reaction system is cooled, a...
Embodiment 2
[0072] Embodiment 2: the preparation of intermediate B
[0073] (1) Synthesis of compound B-2: This step is basically the same as step (2) in Example 1, except that compound B-1 is used instead of A-2, and the yield is 68%.
[0074] (2) Synthesis of compound B-3: under nitrogen protection, add 2.6g zinc powder (0.04mol), a small amount of iodine and 100ml dry DMF in a 250ml reaction flask, add 5g ethyl bromoacetate (0.03mol) after stirring, Heat to 60°C, stir for 3h, filter the solution into another 250ml reaction flask, add 3.68g of compound B-2 (0.01mol) and 0.55g of Pd (PPh 3 ) 4 (5%eq.), heated to 120°C, reacted for 15h. It was extracted with ethyl acetate, separated and dried, and compound B-3 was obtained after column chromatography with a yield of 60%.
[0075] (3) Preparation of Compound B-4: Dissolve 38.2g of Compound B-3 (0.1mol) in 100ml of THF, add 100ml of an aqueous solution containing 12g of LiOH (0.5mol), and stir at room temperature until clear. The volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com