Lipid compound for auxiliary diagnosis of breast cancer and related kit thereof
A compound and breast cancer technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as insufficient research, lack of good diagnostic efficiency of a single lipid species, and no disclosure of lipid compounds for breast cancer, etc., to achieve Strong pertinence, high detection accuracy, and high clinical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Patient Selection
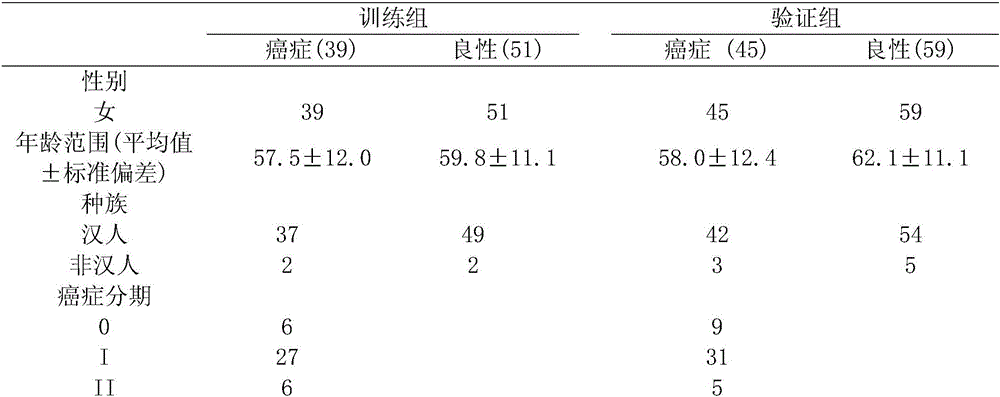
[0032] The training set includes 39 breast cancer and 51 benign breast disease samples obtained from our breast cancer repository. Sample selection should follow the following criteria: (1) all patients were diagnosed and confirmed by pathology; (2) according to the clinical staging method, breast cancer patients should be in the early stage (stage 0, I, II); (3) patients had no other Diseases that may affect lipid metabolism, such as hyperlipidemia, diabetes, and other cancers; (4) All patients were female; (5) Patients did not receive preoperative adjuvant chemotherapy or radiotherapy. Benign breast lesions are defined as hyperplasia, fibromas, cysts, and some unidentified breast diagnoses. Control blood samples were obtained from healthy females with no history of malignancy and inflammation.
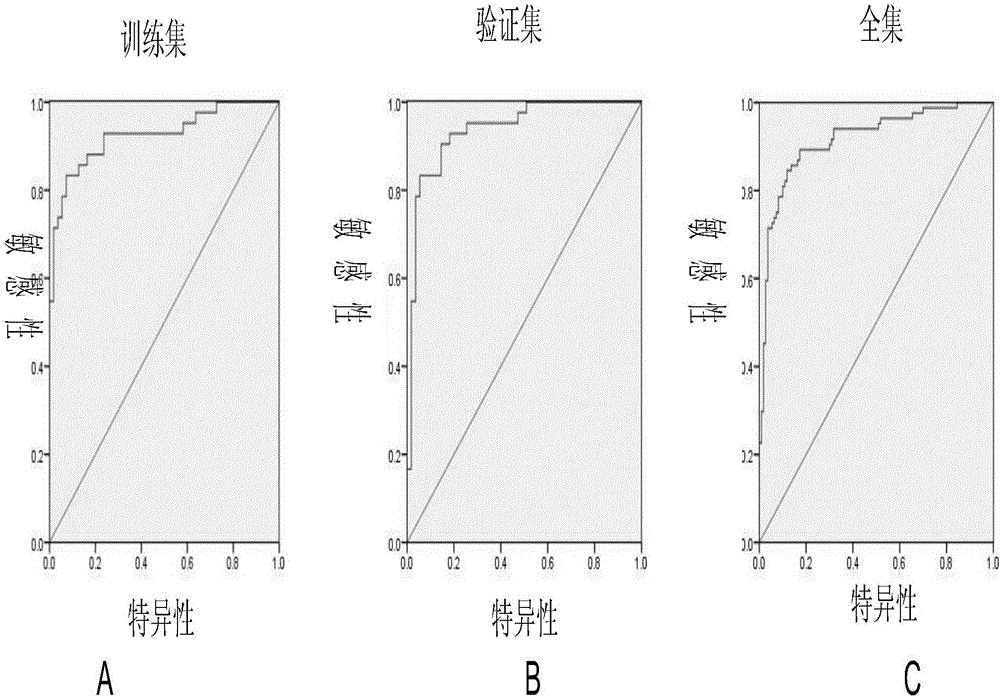
[0033] According to these criteria, plasma samples from 45 patients with early breast cancer (stage 0-II) and 59 patients with benign breast dis...
Embodiment 2
[0034] Embodiment 2: Collection of plasma samples
[0035] Plasma samples were collected after patients had fasted for at least 12 hours. Briefly, plasma collection means that blood is collected into a vacuum blood collection EDTA anticoagulant tube after venipuncture, centrifuged at 4°C and 2600g for 10 minutes within 2 hours, the supernatant is taken, and centrifuged for the second time in the same way, and the plasma is divided into 0.5ml / aliquot Store at -80°C. All plasma samples were shipped on dry ice to the Kansas Lipidomics Research Center for lipid analysis.
[0036] Table 1. Information table of patients with breast cancer and benign breast diseases in the training set and validation set
[0037]
[0038]
Embodiment 3
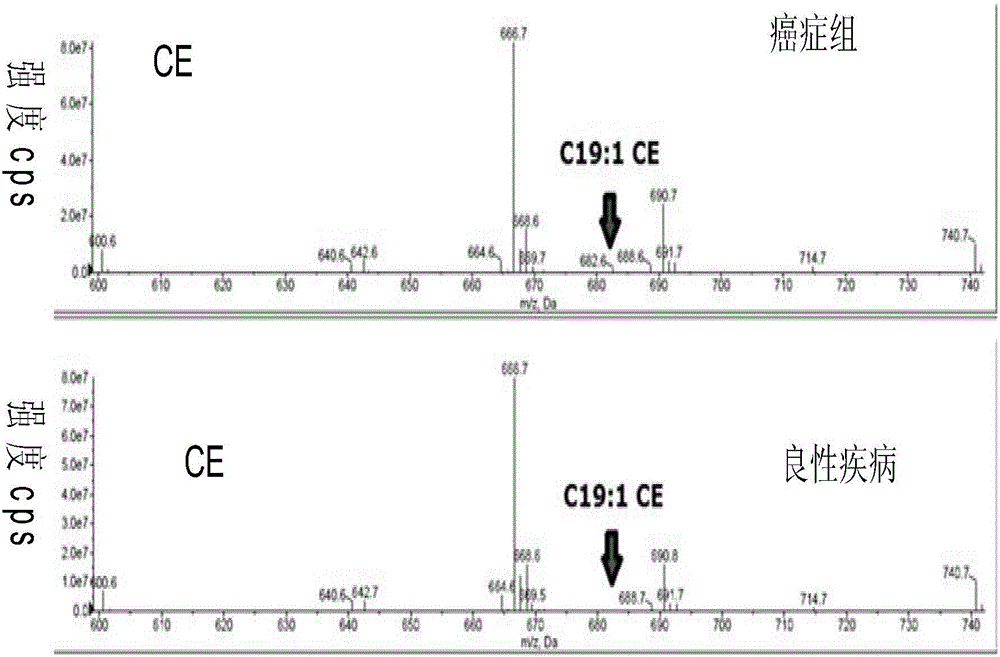
[0039] Example 3: LC-ESI-MS / MS lipid profile
[0040] For each sample analysis, 3 μL of plasma was centrifuged for 20 minutes prior to detection to globularize proteins in surface tube units. In addition, in order to accurately identify all lipids, it is necessary to accurately add internal standards, wherein each type of lipid substance uses two internal standards. After centrifugation for 20 min, lipid extracts were redissolved in solvent and injected into HPLC. The solvent mixing ratio was chloroform / methanol / 300 mM ammonium acetate (μL) 360 / 840 / 44 dissolved in water. All solvents were HPLC grade.
[0041] Lipids were analyzed by triple quadrupole LC-ESI-MS / MS (API 4000, Applied Biosystems, Foster City, CA), which could distinguish their polar heads and chain lengths. Sample introduction is by electrospray ionization (ESI). It reduces ionization suppression effects caused by spectral congestion. Complex lipids generate simple charged ions by ESI, and fragment ions can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com