1,3-dihydroxy-3,7-dimethyl-6-octen-2-one synthesis method
A synthesis method, dihydroxyl technology, applied to compounds of elements of group 4/14 of the periodic table, chemical instruments and methods, hydrolysis to prepare carbonyl compounds, etc., can solve problems such as many steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
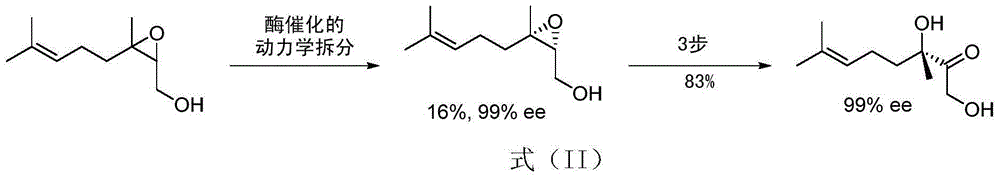
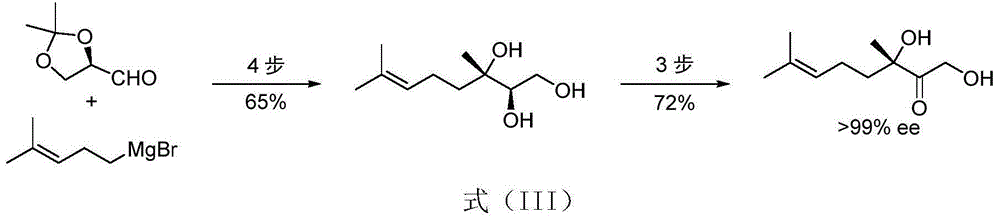
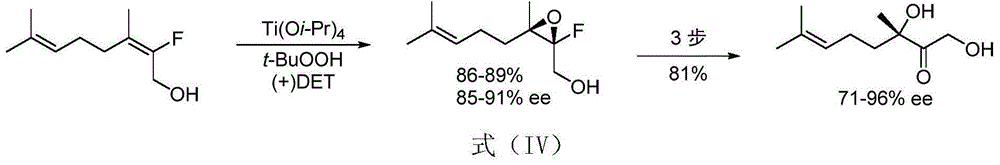
Image
Examples
Embodiment 1-10
[0055] Embodiment 1-10: use TMSCN as cyanide source (table 1):
[0056]
[0057] General operation procedure 1: In a dry 100mL three-necked flask, sequentially add catalyst I, raw material 1 (5.04g, 40mmol), solvent (40mL), and then slowly add TMSCN (6.0mL, 48mmol). The reaction process is monitored by thin-layer chromatography. After the corresponding raw material 1 is consumed, the reaction solution is slowly added dropwise under stirring to a container containing (methanol: 20mL; CH 2 Cl 2 : 60mL; in the Erlenmeyer flask of acid 1), after the dropwise addition, add saturated sodium chloride solution (50mL) again. The obtained two-phase solution was separated with a separatory funnel, the obtained organic phase was washed once with saturated sodium chloride solution (50 mL), and then the solvent was removed by rotary evaporation to obtain a colorless oily liquid. Next, the obtained oily liquid was dissolved in tetrahydrofuran (40mL), base I (50mmol) was added under stir...
Embodiment 11~15
[0063] Embodiment 11~15: use HCN as cyanide source (table 2):
[0064]
[0065] General operating procedure 2: In a 100mL three-neck flask with an absorption device of dry ice and sodium hydroxide, add catalyst I, raw material 1 (5.04g, 40mmol), solvent (40mL) in sequence, and then slowly feed HCN (g ). The reaction process was monitored by thin layer chromatography. After the corresponding starting material 1 was consumed, the solvent was removed by rotary evaporation to obtain a colorless oily liquid. Next, the obtained oily liquid was dissolved in tetrahydrofuran (40mL), base I (50mmol) was added under stirring, the resulting solution was cooled at -10°C for 10min, and then 4 was added slowly. After the dropwise addition, the reaction solution was placed in After stirring at room temperature for 2 h, it was quenched with saturated sodium bicarbonate solution (50 mL). Then, the mixture obtained after removing the solvent by rotary evaporation was subjected to column chr...
Embodiment 16~20
[0070] Embodiment 16~20: use NaCN or KCN as cyanide source (table 3):
[0071]
[0072] General operation procedure 3: In a 100mL three-neck flask equipped with an absorption device of dry ice and sodium hydroxide, add catalyst Ⅰ, raw material 1 (5.04g, 40mmol), solvent (40mL) in sequence, and then add NaCN or KCN. The reaction process was monitored by thin-layer chromatography. After the corresponding raw material 1 was consumed, the solvent was removed by suction filtration and rotary evaporation to obtain a colorless oily liquid. Next, the obtained oily liquid was dissolved in tetrahydrofuran (40mL), base I (50mmol) was added under stirring, the resulting solution was cooled at -10°C for 10min, and then 4 (50mmol) was slowly added. After the dropwise addition, The reaction solution was stirred at room temperature for 2 h and quenched with saturated sodium bicarbonate solution (50 mL). Then, the mixture obtained after removing the solvent by rotary evaporation was subjec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com