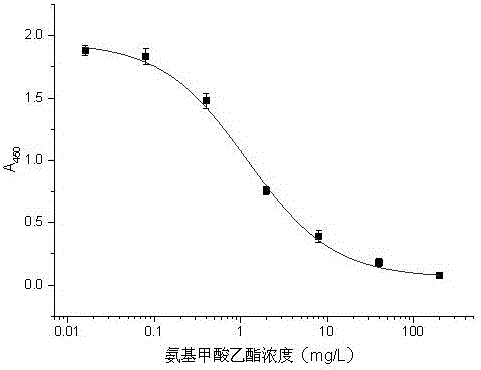
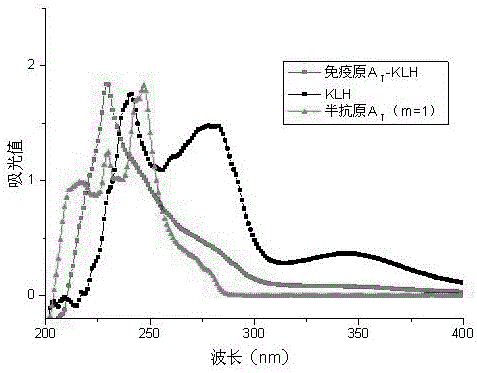
Urethane hapten composition and artificial antigen composition, and preparation methods and application thereof
A technology of urethane and artificial antigen, which is applied in the field of artificial antigen combination and its preparation, and the field of urethane hapten combination, which can solve the problems of relying on large-scale instruments, high detection costs, and complicated steps, so as to improve detection efficiency, Effect of increasing molecular weight and aromaticity and reducing detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
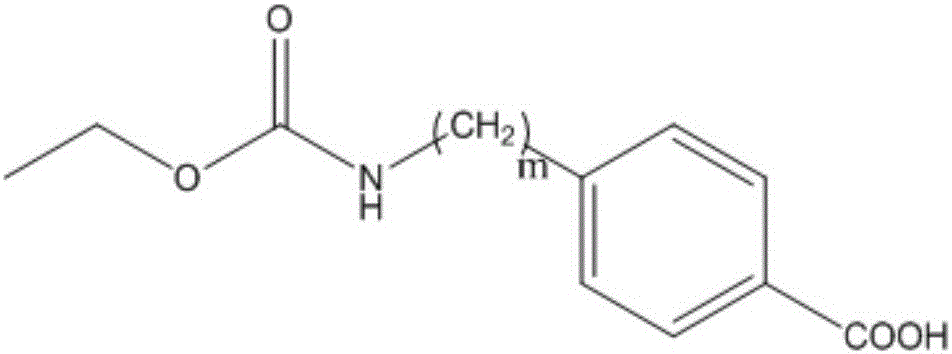
[0039] Example 1 Preparation method of urethane hapten AⅠ (m=0)
[0040] Dissolve 1.37g of 4-aminobenzoic acid in 30mL of 1mol / L sodium bicarbonate solution, add 2mL of diethyl pyrocarbonate dropwise to the solution, stir and react at room temperature overnight, after the reaction, adjust the acidity of the reaction mixture to pH 1 to 2, there is a white precipitate, filter the precipitate, wash and dry with distilled water to obtain 1.78g of white solid, which is hapten A Ⅰ (m=0). ESI-MS analysis(negative)m / z 190[M-H] – ; 1 H NMR (600MHz, MeOD) δ7.97–7.93(m, 2H), 7.55(d, J=8.8Hz, 2H), 4.21(q, J=7.1Hz, 2H), 1.32(t, J=7.1Hz ,3H).
Embodiment 2
[0041] Example 2 Preparation method of urethane hapten AⅠ (m=1)
[0042] Dissolve 1.51g of 4-aminomethylbenzoic acid in 30mL of 1mol / L sodium bicarbonate solution, add 2mL of diethyl pyrocarbonate dropwise to the solution, stir and react at room temperature overnight, after the reaction, adjust the acidity of the reaction mixture to When the pH is 1-2, there is a white precipitate, filter the precipitate, wash with distilled water and dry to obtain 2.04g of white solid which is hapten A Ⅰ (m=1). ESI-MS analysis(negative)m / z 222[M-H] – ; 1H NMR (600MHz, MeOD) δ7.99(d, J=8.2Hz, 2H), 7.39(d, J=8.1Hz, 2H), 4.35(s, 2H), 4.12(q, J=7.1Hz, 2H ),1.26(t,J=7.1Hz,3H).
Embodiment 3
[0043] Example 3 Preparation method of urethane hapten AⅠ (m=2)
[0044] Dissolve 1.65g of 4-(2-aminoethyl)benzoic acid in 30mL of 1mol / L sodium bicarbonate solution, add 2mL of diethyl pyrocarbonate dropwise to the solution, stir and react at room temperature overnight, after the reaction is completed, the reaction Adjust the acidity of the mixed solution to a pH of 1-2, and a white precipitate precipitates out, filter the precipitate, wash with distilled water and dry to obtain 2.13 g of a white solid, which is Hapten A Ⅰ (m=2). ESI-MS analysis (negative) m / z 236[M-H] – ;1H NMR (600MHz, MeOD) δ7.96(d, J=8.2Hz, 2H), 7.33(d, J=8.1Hz, 2H), 4.06(q, J=7.1Hz, 2H), 3.36(t, J=7.3Hz, 2H), 2.86(t, J=7.3Hz, 2H), 1.22(t, J=7.1Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com