Preparation method of pure-phase bismuth ferrite
A technology of bismuth ferrite and powder, which is applied in the direction of chemical instruments and methods, iron compounds, nanotechnology for materials and surface science, etc., can solve the problems that hinder the application of bismuth ferrite materials, complicated process, pure phase bismuth ferrite Problems such as large leakage current have achieved significant technological progress, simple process flow, and easy-to-control reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 40.4024g Fe(NO 3 ) 3 9H 2 O and 48.5102g Bi(NO 3 ) 3 ·5H 2 o
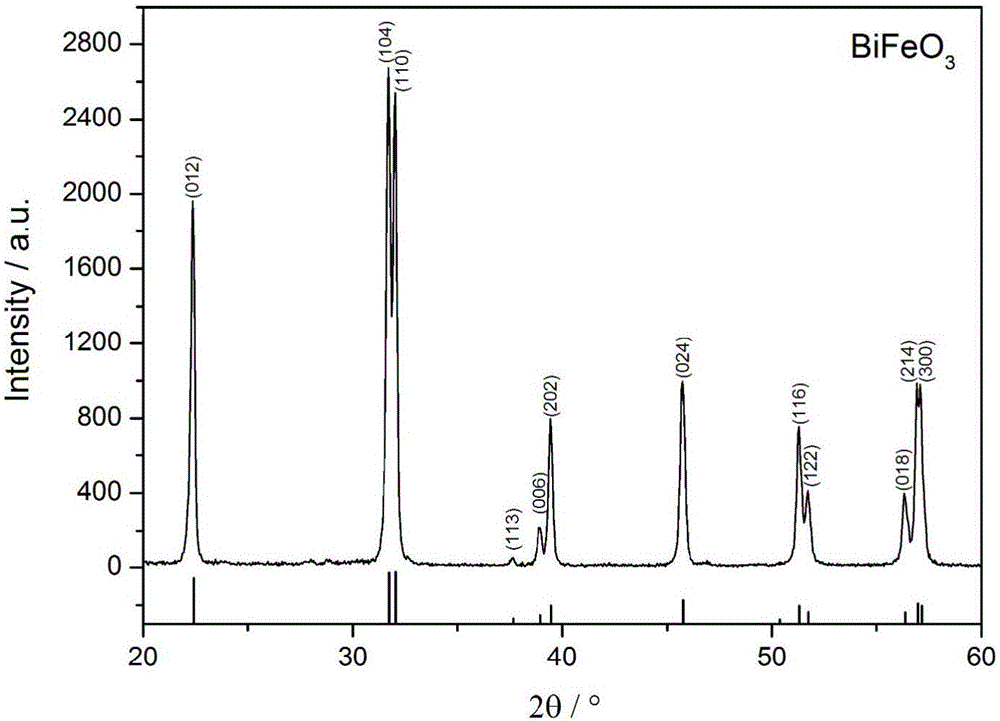
[0023] Dissolve in 1L of HNO with a concentration of 1mol / L 3 Prepare a precursor solution with a metal ion concentration of 0.2 mol / L in the solution, and slowly and evenly add the precursor solution dropwise to 200 mL of NH with a volume ratio of 1:2 under magnetic stirring. 3 ·H 2 O / NH 4 HCO 3 Mixed precipitant (NH 4 HCO 3 concentration is 0.1mol / L), when the pH value of the titration system is 9.5, continue to stir for 20 minutes, let stand and age for 16 hours, filter the precipitate obtained from the reaction, wash with deionized water until neutral, and then use absolute ethanol Wash 3 times, dry in a drying oven at 50°C to obtain the precursor powder, then place the precursor powder in a muffle furnace, heat up to 600°C at a rate of 10°C / min for calcination for 1 hour, and cool to obtain pure-phase bismuth ferrite Powder.
[0024] The XRD characterization figure of the pure-phase b...
Embodiment 2
[0027] Weigh 40.4024g Fe(NO 3 ) 3 9H 2 O and 48.5102g Bi(NO 3 ) 3 ·5H 2 o
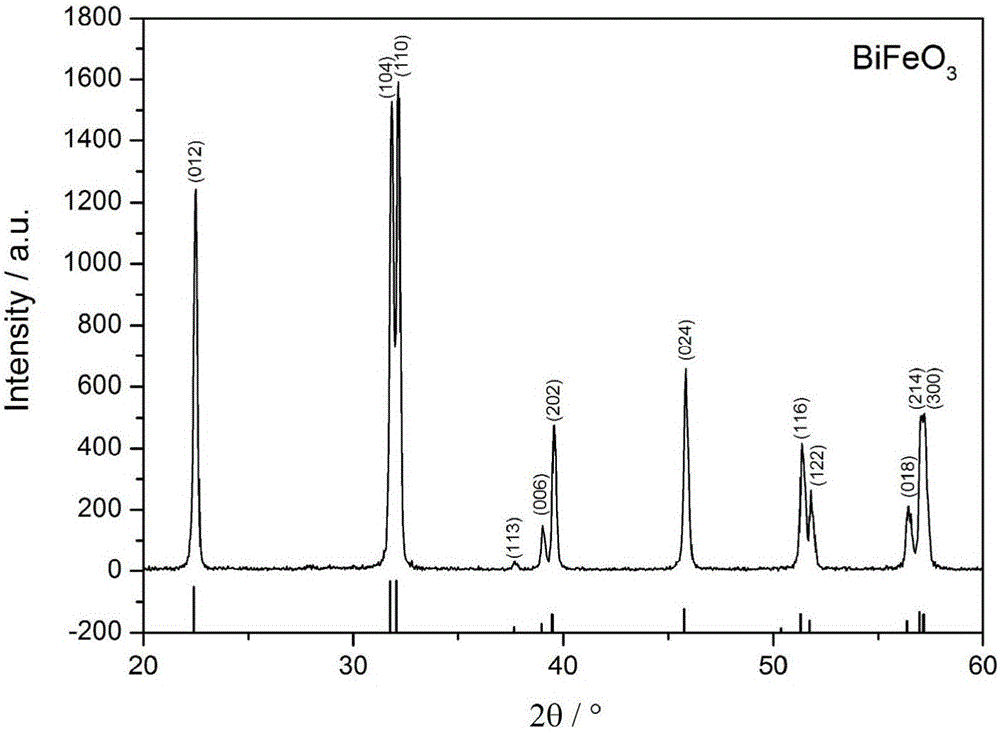
[0028] Dissolve in 1L of HNO with a concentration of 1mol / L 3 Prepare a precursor solution with a metal ion concentration of 0.2 mol / L in the solution, and slowly and evenly add the precursor solution dropwise to 200 mL of NH with a volume ratio of 1:2 under magnetic stirring. 3 ·H 2 O / NH 4 HCO 3 Mixed precipitant (NH 4 HCO 3 concentration is 0.1mol / L), when the pH value of the titration system is 10, continue to stir for 20 minutes, let it stand for 20 hours, and filter the precipitate obtained from the reaction, wash it with deionized water until it is neutral, and then wash it with absolute ethanol Wash 3 times, dry in a drying oven at 50°C to obtain the precursor powder, then place the precursor powder in a muffle furnace, heat up to 600°C at a rate of 10°C / min for calcination for 2 hours, and cool to obtain pure-phase bismuth ferrite Powder.
[0029] The XRD characterization figure of...
Embodiment 3
[0032] Weigh 40.4024g Fe(NO 3 ) 3 9H 2 O and 48.5102g Bi(NO 3 ) 3 ·5H 2 o
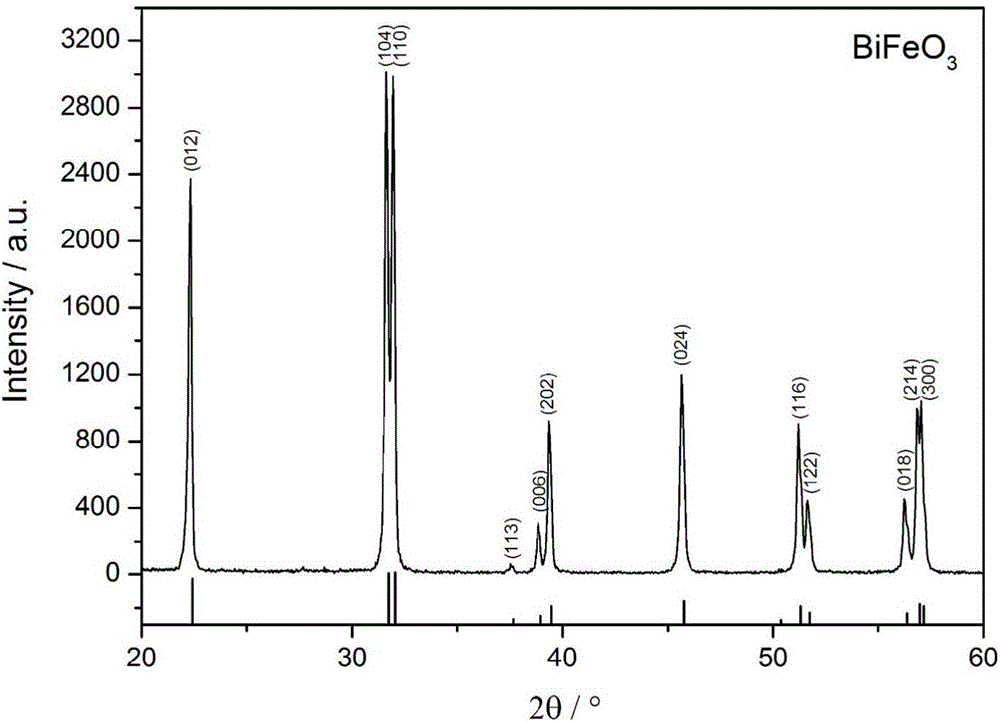
[0033] Dissolve in 1L of HNO with a concentration of 1mol / L 3 Prepare a precursor solution with a metal ion concentration of 0.2 mol / L in the solution, and slowly and evenly add the precursor solution dropwise to 200 mL of NH with a volume ratio of 1:2 under magnetic stirring. 3 ·H 2 O / NH 4 HCO 3 Mixed precipitant (NH 4 HCO 3 Concentration is 0.1mol / L), when the pH value of the titration system is 9, continue to stir for 20 minutes, let stand and age for 24 hours, the precipitate obtained from the reaction is filtered, washed with deionized water until neutral, and then washed with absolute ethanol Wash twice, dry in a drying oven at 60°C to obtain the precursor powder, then place the precursor powder in a muffle furnace, heat up to 600°C at a rate of 10°C / min for calcination for 3 hours, and cool to obtain pure-phase bismuth ferrite Powder.
[0034] The XRD characterization figure of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com