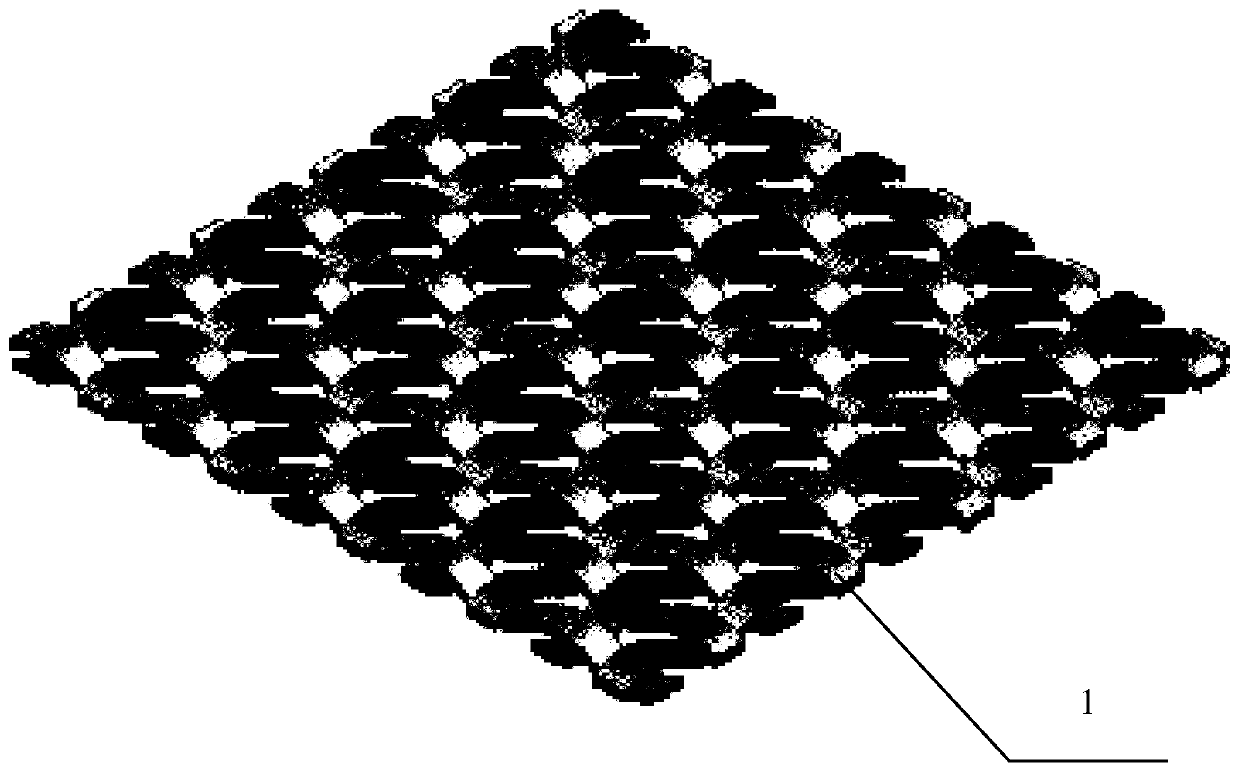
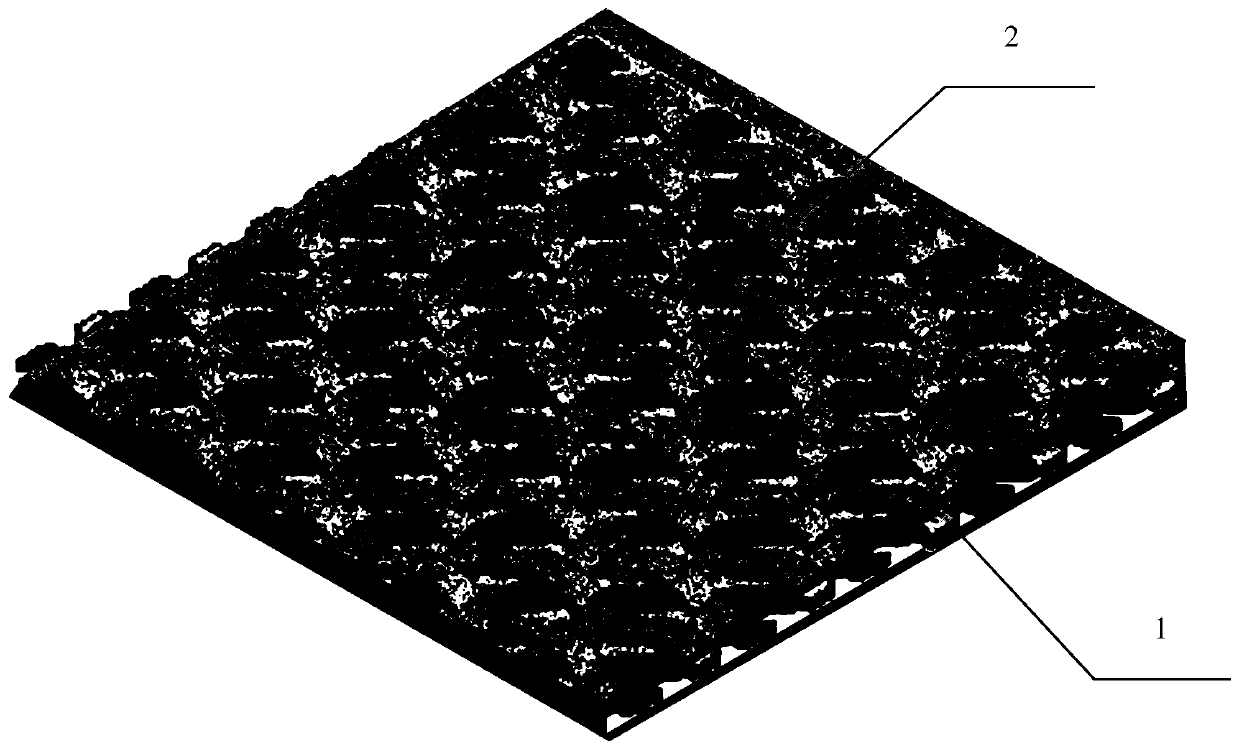
A kind of preparation method of composite artificial dura mater
An artificial dura mater, composite technology, applied in the field of biomedical materials, can solve the problems of scarring or wrapping reaction in the retention time, increasing the time of surgery and treatment, weak biocompatibility, etc. Industrial preparation, the effect of increasing fiber surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) select fibrin and deionized water to configure the hydrogel solution according to the molar ratio of 1:10;
[0030] 2) making the fibroblast growth factor and the hydrogel material into a suspension according to the molar ratio of 1:5000;
[0031] 3) Place the hydrogel suspension in a film-forming mold to form a film with a thickness of 100 μm, and cure at 4°C for 2 hours;
[0032] 4) The braided wire is made of PLA, and the surface is treated with helium plasma, the gas temperature is 90°C, the flow rate is 4L / min, and the treatment time is 4min;
[0033] 5) The braided wire is made of the Heglis braiding process to make the braided layer with a thickness of 500 μm;
[0034] 6) After the braided layer is laid on the cured hydrogel, a layer of hydrogel suspension with a thickness of 100 μm is laid on the braided layer, and cured at 4°C for 2 hours;
[0035] 7) The artificial dura mater is sterilized with a radiation dose of 25kGy.
Embodiment 2
[0037] 1) Select collagen and deionized water to configure the hydrogel solution according to the molar ratio of 1:20;
[0038] 2) making the fibroblast growth factor and the hydrogel material into a suspension according to the molar ratio of 1:4000;
[0039] 3) Place the hydrogel suspension in a film-forming mold to form a film with a thickness of 200 μm, and cure at 4°C for 3 hours;
[0040] 4) The braided wire is made of PGA, and the surface is treated with helium plasma, the gas temperature is 100°C, the flow rate is 6L / min, and the treatment time is 6min;
[0041] 5) The braided wire is made of the Heglis braiding process to make the braided layer with a thickness of 1000 μm;
[0042] 6) After the braided layer is laid on the cured hydrogel, a layer of hydrogel suspension with a thickness of 200 μm is laid on the braided layer, and cured at 4°C for 3 hours;
[0043] 7) The artificial dura mater is sterilized by giving the irradiation dose of 28kGy.
Embodiment 3
[0045] 1) select collagen and deionized water to configure hydrogel solution according to the molar ratio of 1:20;
[0046] 2) The vascular endothelial growth factor and the hydrogel material are made into a suspension according to the molar ratio of 1:4000;
[0047] 3) Place the hydrogel suspension in a film-forming mold to form a film with a thickness of 300 μm, and cure at 6°C for 3 hours;
[0048] 4) The braided wire is made of PLGA, and the surface is treated with oxygen plasma, the gas temperature is 100°C, the flow rate is 8L / min, and the treatment time is 8min;
[0049] 5) The braided wire is made of a two-dimensional three-axis braiding process to make a braided layer with a thickness of 1000 μm;
[0050] 6) After the braided layer is laid on the cured hydrogel, a layer of hydrogel suspension with a thickness of 300 μm is laid on the braided layer, and cured at 6°C for 3 hours;
[0051] 7) The artificial dura mater is sterilized by giving the irradiation dose of 30k...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com