Preparation method for ultrapure ultrafine cobalt carbonate
A technology of cobalt carbonate and carbonate, applied in directions such as cobalt carbonate, can solve the problems of high processing cost, difficult to wash, difficult to handle impurities, etc., and achieve the effect of simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
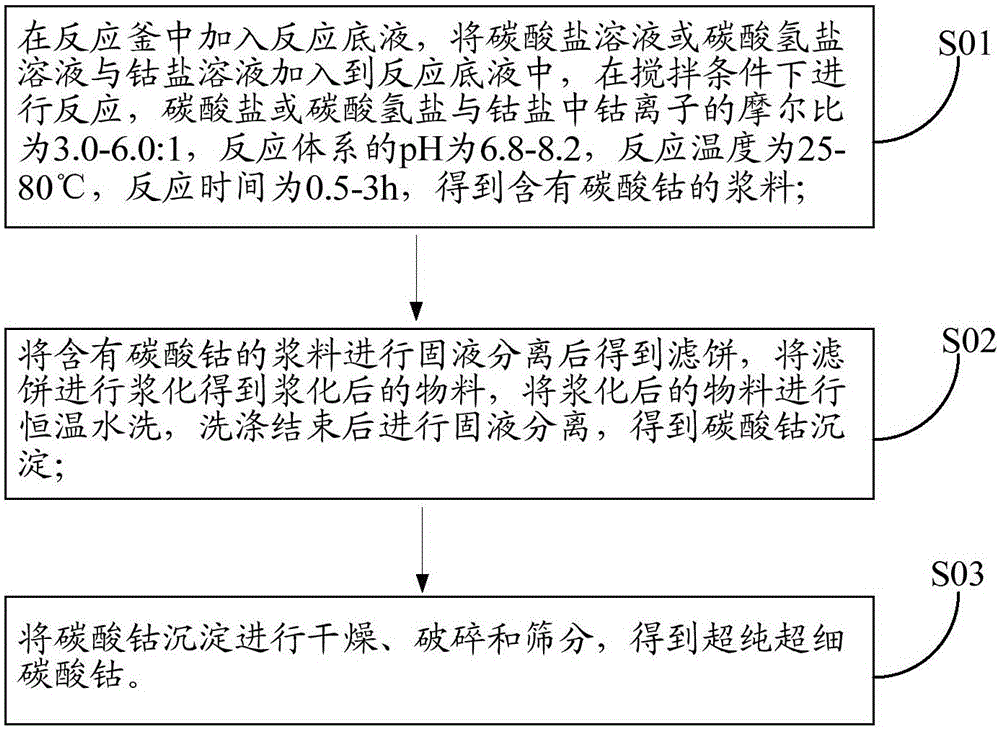
[0066] Please refer to figure 1 , figure 1 It is a schematic flow sheet of an ultra-pure ultra-fine cobalt carbonate preparation method in an embodiment of the present invention, comprising the following steps:
[0067] S01. Add the reaction bottom liquid in the reaction kettle, add the carbonate solution or bicarbonate solution and the cobalt salt solution to the reaction bottom liquid, and react under stirring conditions, the carbonate or bicarbonate and the cobalt salt The molar ratio of cobalt ions is 3.0-6.0:1, the pH of the reaction system is 6.8-8.2, the reaction temperature is 25-80°C, and the reaction time is 0.5-3h to obtain a slurry containing cobalt carbonate;
[0068] S02, performing solid-liquid separation on the slurry containing cobalt carbonate to obtain a filter cake, slurrying the filter cake to obtain a slurried material, washing the slurried material with water at a constant temperature, and performing solid-liquid separation after washing to obtain Coba...
Embodiment 1
[0074] A preparation method of ultrapure ultrafine cobalt carbonate, comprising the following steps:
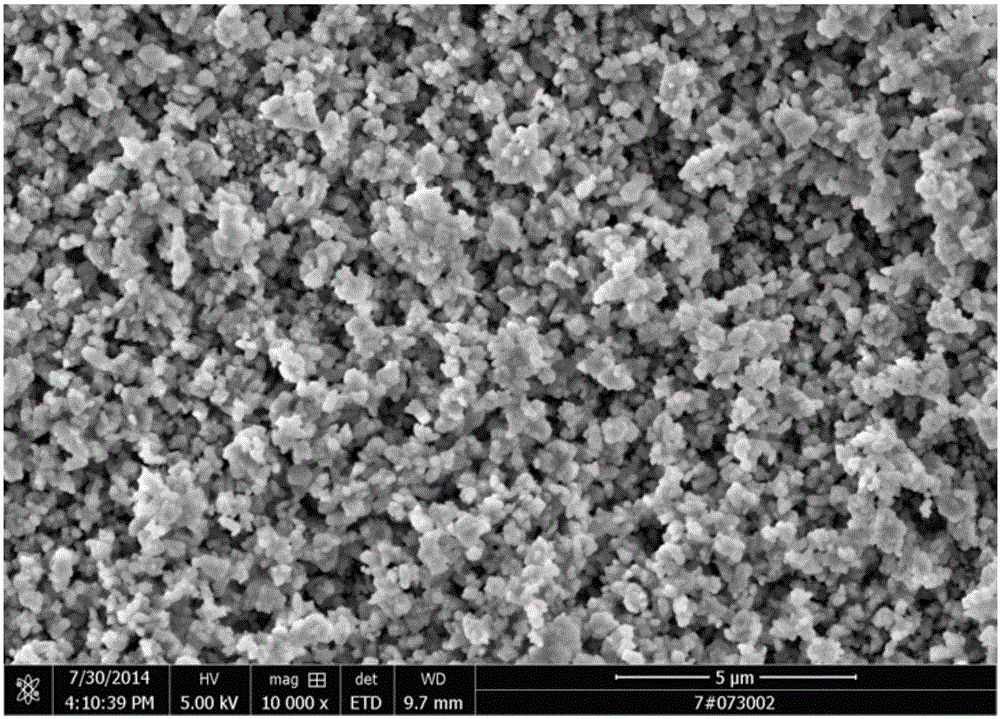
[0075] (1), add the ammonium bicarbonate solution (reaction bottom liquid) that concentration is 40g / L to stirring paddle place in synthetic reactor, after raising reactor temperature to 35 ℃, start stirring paddle, control rotating speed is 250rpm, simultaneously Cobalt chloride solution and ammonium bicarbonate solution are added in parallel, wherein the flow rate of cobalt chloride solution is 400L / h, the pH is adjusted by the ammonium bicarbonate flow rate, the reaction pH is controlled between 6.8-7.8, and the reaction temperature is 35°C. Stop feeding after reacting 2h, obtain the slurry that contains cobalt carbonate; The mol ratio of cobalt ion in ammonium bicarbonate and cobalt chloride is 4.5:1;
[0076] (2) The slurry containing cobalt carbonate is transported to the plate and frame filter press for solid-liquid separation, and the pressure is 22Mpa to obtain mothe...
Embodiment 2
[0083] A preparation method of ultrapure ultrafine cobalt carbonate, comprising the following steps:
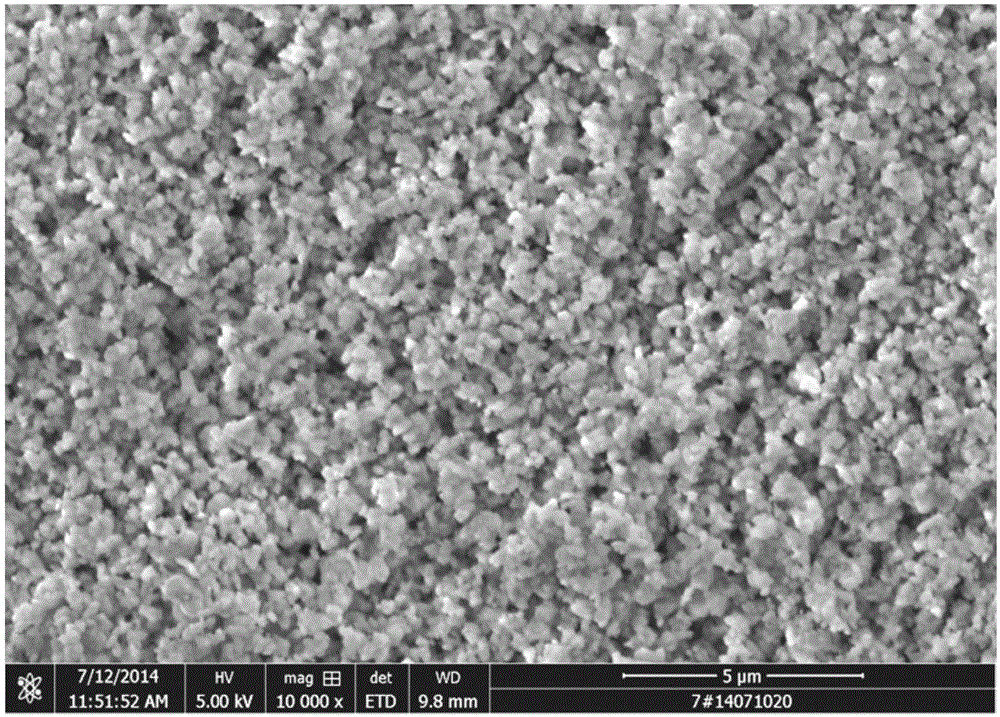
[0084] (1) Add pure water (reaction bottom liquid) to the stirring paddle in the synthesis reactor, raise the temperature of the reactor to 50°C, start the stirring paddle, control the speed at 500rpm, and simultaneously add cobalt chloride solution and carbonic acid Ammonium solution, wherein the flow rate of the cobalt chloride solution is 300L / h, the pH is adjusted by the ammonium carbonate flow rate, the reaction pH is controlled between 7.3-8.2, and the reaction temperature is 50°C. Stop feeding after reacting 1h, obtain the slurry that contains cobalt carbonate; The mol ratio of cobalt ion in ammonium carbonate and cobalt chloride is 6.0:1;
[0085] (2) The slurry containing cobalt carbonate is transported to the microporous filter for solid-liquid separation, the pressure is 0.2Mpa, mother liquor and filter cake are obtained, the mother liquor enters the recovery syste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com