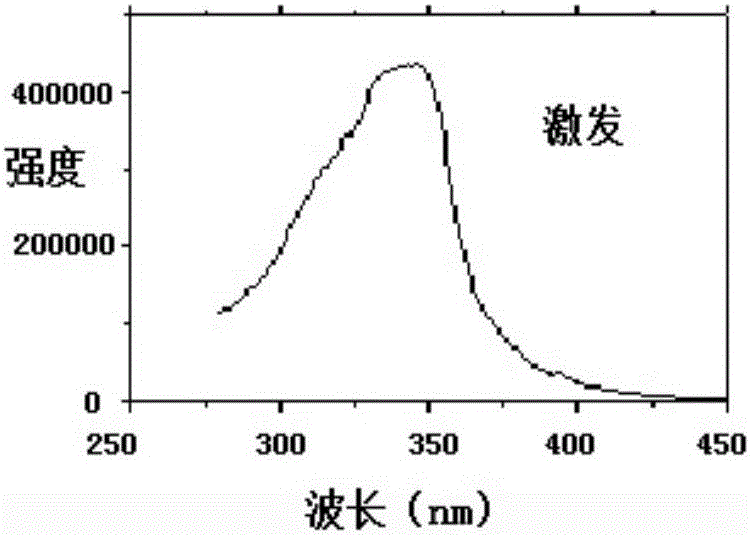
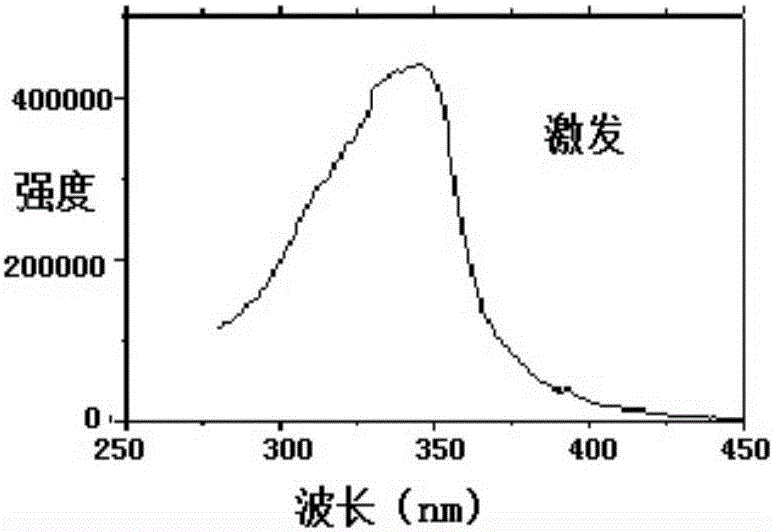
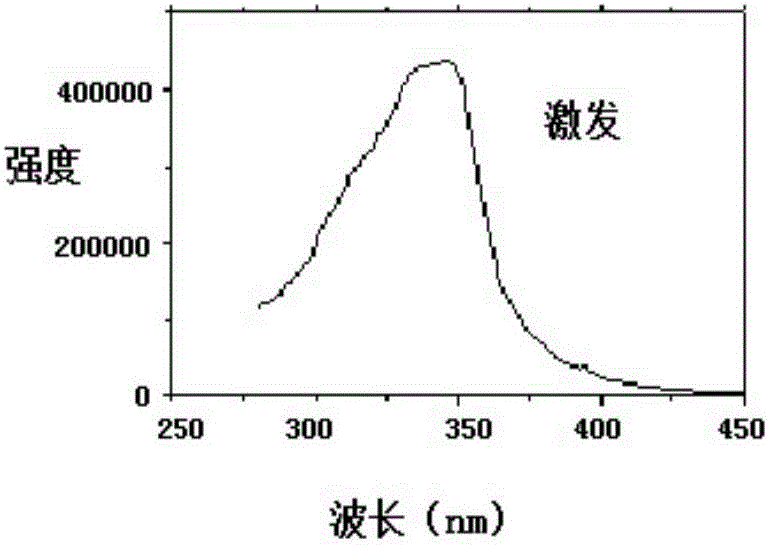
Lanthanide metal ether complex and preparation method and application thereof
A technology of lanthanide metals and complexes, which is applied in the field of lanthanide metal cryptate complexes and their preparation, and can solve the problems of short fluorescence lifetime, weak fluorescence intensity, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1 The preparation of the intermediate shown in formula (Ⅳ)
[0102] References Helvetica Chimica Acta, 1992, 75, 1578.
[0103]
[0104]Under anhydrous and oxygen-free conditions, first take 1 mmol of 6,6-dimethyl-4,4-dimethyl ester-2,2-bipyridine (ie: the compound shown in formula (1-1)) and add it to In a 100mL two-necked flask, add about 40mL of anhydrous and oxygen-free toluene, heat to 80°C, add t-BuLi solution in t-BuOH dropwise; after the addition, continue to reflux for 30min to obtain compound 6,6- Methyl-4,4-di-tert-butyl-2,2-bipyridine (namely: the intermediate represented by formula (1-2)), the yield is 58%.
[0105] First take 0.5mmol of 6,6-dimethyl-4,4-di-tert-butyl-2,2-bipyridine (ie: the compound shown in formula (1-2)) into the round bottom flask, and then sequentially Join CCl 4 , 2.1mmol NBS and a catalytic amount of diethyl phosphite, under the conditions of anhydrous, anaerobic and argon protection, heated and refluxed overnight; af...
Embodiment 2
[0110] Example 2 The preparation of the intermediate shown in formula (Ⅴ)
[0111] References J.Am.Chem.Soc.2010, 132, 14334.
[0112]
[0113] Take 0.3mmol of the compound represented by formula (1-3), add it to 3mL of chloroform solution, heat to reflux until it becomes completely clear, then slowly add 0.66mmol of hexamethylenetetramine in chloroform solution (1.5mL×2) , and then continue to heat and reflux for 3h; after the reaction is over, let it stand, cool, filter, and the filter cake is washed with chloroform and vacuum filtered; then, add H to the filter cake 2 O: EtOH: 47% HBr aqueous solution with a volume ratio of 1.4:5.8:1.0 mixed solvent, heat and stir at 75°C until the solids are completely dissolved, and then the solution becomes clear; stop the reaction, cool and stand for 1h, then ice bath for 30min , pale yellow crystals were precipitated, filtered, the filter cake was washed with ethanol, and vacuum filtered to obtain the intermediate shown in formul...
Embodiment 3
[0118] Example 3 The preparation of the intermediate shown in formula (Ⅲ)
[0119]
[0120] In an anhydrous, anaerobic two-necked flask, add 300mL of acetonitrile, then sequentially add 20mmol of the compound shown in formula (IV), 10mmol of the compound shown in formula (V) and 0.26mmol of sodium carbonate, heat and reflux for 2 to 3 days , after the reaction was finished, let stand, cool, filter, the filtrate was concentrated under reduced pressure, and then washed with ether to obtain the intermediate shown in formula (Ⅲ), with a yield of 48%.
[0121] The structure confirmation data of the intermediate represented by formula (Ⅲ):
[0122] 1 H NMR (Acetone-d 6 ):8.73(s,1H,Hbpy),8.28(m,2H,Hbpy),7.76(s,2H,Hbpy),7.58(s,1H,Hbpy),3.95(s,9H,-OCH 3 ),1.54(s,9H,-C(CH 3 ) 3 );
[0123] MS: 557..23(C 60 h 66 NaN 8 o 12 +H + ) 2+ (M / z=557.23);
[0124] Elemental analysis (%): C, 62.68; H, 5.79; N, 9.75.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com