Enhanced polysaccharide antigen immunogenic protein carrier as well as preparation method and application thereof
A polysaccharide antigen and protein carrier technology, applied in the field of polysaccharide protein conjugate vaccine development, can solve the problems of difficult quality control, large molecular weight of TT, residual toxicity of toxoid, etc., and achieve easy quality control, reduce immune interference, and enhance immunogenicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of protein carrier CRM197A-Hin47
[0039] Step 1: Construction of CRM197A-Hin47 expression vector
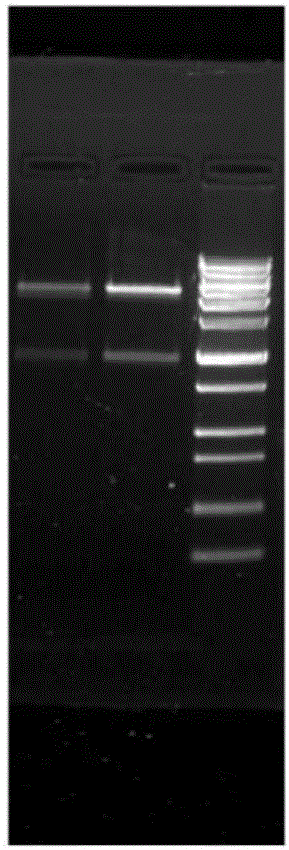
[0040] Using the CRM197A and Hin47 gene sequences as templates and G4S as a linker, the target gene of the fusion protein can be amplified by over-lap PCR, such as figure 1 as shown, figure 1 It is the agarose gel electrophoresis image of over-lap PCR amplification product.
[0041] Both the fusion gene and the pET 9a expression vector were double-digested with BamHI restriction enzyme and NdeI restriction endonuclease, and then ligated by T4 DNA ligase, overnight at 16°C; the recombinant expression vector was transformed into DH5a competent, picked Single clone, inoculated in 5ml LB medium, cultured overnight at 37°C 200rpm; extract the plasmid for enzyme digestion identification, the verification results are as follows figure 2 as shown, figure 2 Indicates that CRM197A is successfully linked to the Hin47 gene.
[0042] The amino acid composit...
Embodiment 2
[0060] Example 2: Study on Immunogenicity of CRM197A-Hin47 Fusion Protein
[0061] Select 100 to SPF grade 10-12g female BALB / c mice for immunogenicity research, the method is as follows: the mice are randomly divided into 10 groups, 10 in each group. Abdominal subcutaneous immunization, the volume of immunization is 0.2mL, and the composition and content information of mice in each group are shown in Table 3. Immunization was carried out on the 0th and 14th day respectively. On the 28th day, the eyeballs were picked to collect whole blood, and the serum was collected by centrifugation at 8000rpm for 6 minutes, and stored at -20°C. The antibody titers against Hin47 and CRM197A proteins in mouse serum were determined by indirect ELISA.
[0062] Table 2 Components and contents of mice immunization in each group
[0063] Group No Sample serial number immune dose 1 Hin47-1 1μg 2 Hin47-2 5μg 3 Hin47-3 10μg 4 CRM197A-1 1μg 5 CRM1...
Embodiment 3
[0067] Example 3: Preparation of CRM197A-Hin47-PRP conjugate vaccine
[0068] Step 1: Covalent binding of Haemophilus influenzae type b capsular polysaccharide PRP to CRM197A-Hin47
[0069] Prepare Haemophilus influenzae type b capsular polysaccharide with common laboratory method, get appropriate polysaccharide and be dissolved in purified water (10mg / ml), 1-cyano group-4-dimethylamino-pyridine tetrafluoroboric acid (1-Cyano -4-dimethylaminopyridiniumtetrafluoroborate, CDAP) was dissolved in acetonitrile (100mg / ml), and CDAP was added at a ratio of 0.75mg CDAP / mg to activate the reaction for 30s. Then add 0.2M TEA (10ul / mg), adjust the pH value to 9.5, add fusion protein CRM197A-Hin47 (5mg / ml) after 150s, react at room temperature for 1h, react overnight at 4°C, add 5 drops of 2M glycine solution to terminate the binding reaction. After diafiltration, separate and purify.
[0070] Step 2: Purification of CRM197A-Hin47-PRP
[0071] The conjugate was separated and purified o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com