A kind of Eu-doped aluminosilicate fluorescent material with high-efficiency blue light emission and preparation method thereof
A fluorescent material, aluminosilicate technology, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of expensive preparation facilities, uneven atmosphere contact, safety problems of reducing gas, etc., and achieve good ultraviolet absorption and product physicochemical The effect of stable performance and safety guarantee
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
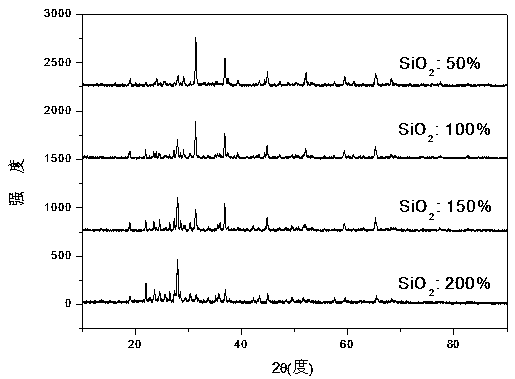
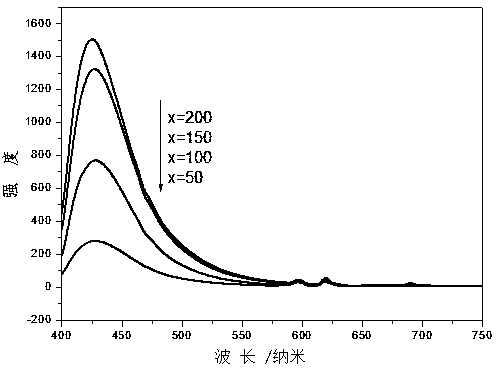
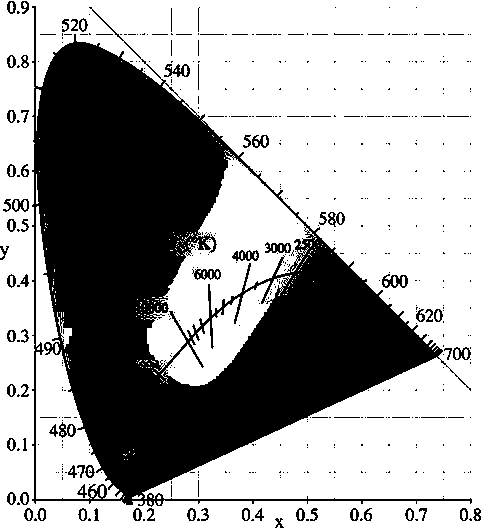
[0023] Weigh (MgCO 3 ) 4 Mg(OH) 2 • 5H 2 O: 0.4879g, Al 2 o 3 : 0.5121 g, Eu 2 o 3 : 0.0177 g, SiO 2 : 0.1509 g Total 1.1608 g. In this embodiment, the heat treatment temperature is 1000° C., and the reaction time is 20 h. X-ray diffraction results (such as figure 1 shown) shows that the sample is mainly MgAl 2 o 4 with a small amount of Mg 2 Al 2 SiO 7 Mutually. After grinding, measure its emission spectrum at room temperature with F-4600 fluorescence spectrophotometer (e.g. figure 2 shown). Under the excitation of 365nm near-ultraviolet light, Eu was detected 2+ : 5 d →4 f slightly stronger broadband emission, while Eu 3+ : 5 D. 0 − 7 f J (J = 0,1,2,3,4) the emission intensity is very weak, indicating that the incorporated Eu 3+ Partially self-reduced to Eu 2+ , and its emission spectrum color coordinate position is: x=0.1782 y=0.0868 (such as image 3 ), located in the blue light area, the fluorescent material emits a slightly stronger blue ligh...
Embodiment 2
[0025] Weigh (MgCO 3 ) 4 Mg(OH) 2 • 5H 2 O: 0.4879g, Al 2 o 3 : 0.5121 g, Eu 2 o 3 : 0.0177 g, SiO 2 : 0.3017 g, a total of 1.3194 g. In this embodiment, the heat treatment temperature is 1100° C., and the reaction time is 15 hours. X-ray diffraction results (such as figure 1 shown) shows that the sample except MgAl 2 o 4 with Mg 2 Al 2 SiO 7 A new phase MgAl 2 Si 2 o 8 . After grinding, measure its emission spectrum at room temperature with F-4600 fluorescence spectrophotometer (e.g. figure 2 shown). Under the excitation of 365nm near-ultraviolet light, Eu was detected 2+ : 5 d →4 f Strong broadband emission, while Eu 3+ : 5 D. 0 − 7 f J (J = 0,1,2,3,4) the emission intensity is very weak, indicating that the incorporated Eu 3+ More has been self-reduced to Eu 2+ , and its emission spectrum color coordinate position is: x=0.1681 y=0.0771 (such as image 3 ), located in the blue light region with a shorter wavelength, and the fluorescent materia...
Embodiment 3
[0027] Weigh (MgCO 3 ) 4 Mg(OH) 2 • 5H 2 O: 0.4879g, Al 2 o 3 : 0.5121 g, Eu 2 o 3 : 0.0177 g, SiO 2 : 0.4526 g Total 1.4703 g. In this embodiment, the heat treatment temperature is 1300° C., and the reaction time is 10 h. X-ray diffraction results (such as figure 1 shown) shows that the sample MgAlO 4 Phase reduction, while Mg 2 Al2 SiO 7 , MgAl 2 Si 2 o 8 phase increase. After grinding, measure its emission spectrum at room temperature with F-4600 fluorescence spectrophotometer (e.g. figure 2 shown). Under the excitation of 365nm near-ultraviolet light, Eu was detected 2+ : 5 d →4 f Strong broadband emission, while Eu 3+ : 5 D. 0 − 7 f J (J = 0,1,2,3,4) the emission intensity is very weak, indicating that the incorporated Eu 3+ A large fraction is self-reduced to Eu 2+ , its emission spectrum color coordinate position is: x= 0.1632 y= 0.0772 (such as image 3 ), located in the blue light area, the fluorescent material emits strong blue light vis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com