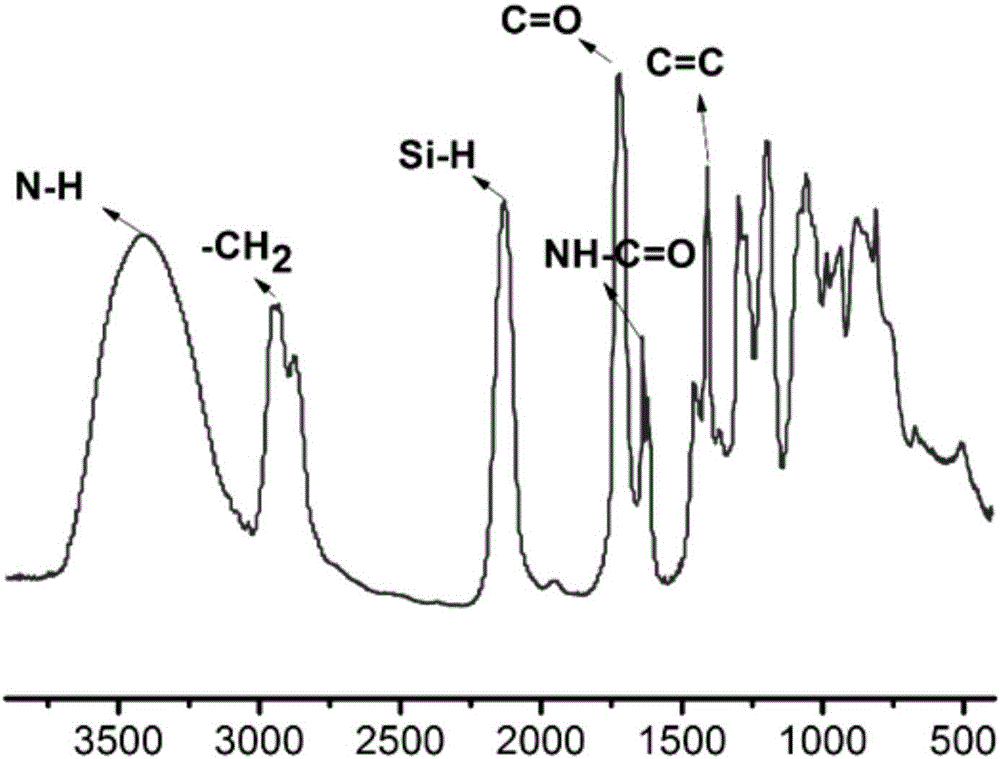
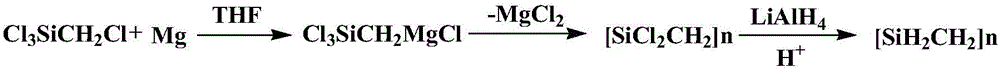Preparation method for liquid hyperbranched polycarbosilane containing acryloyloxy group
An acryloxy, polycarbosilane technology, which is applied in the field of preparation of liquid hyperbranched polycarbosilane, can solve the problems of high content of unsaturated double bonds, difficult to control, and low yield of LHBPCS ceramicization, and achieves a simple preparation process. , The effect of improving the ceramic yield and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Synthesis of LHBPCS: Add 500ml THF solvent, 40.84g magnesium powder and two iodine simple substances into the reaction kettle, then add 264.84g Cl 3 SiCH 2 Cl, where magnesium and Cl 3 SiCH 2 The molar ratio of Cl is 1.05:1. After the dropwise addition, react at 60°C for 12h; then add 41.37g lithium aluminum hydride for reduction at 60°C. After the reduction, add 68.54g, 4mol / L hydrochloric acid to quench the remaining Magnesium powder and lithium aluminum hydride, and finally filtered, pickled, washed with water, and vacuum distilled to obtain LHBPCS containing Si-H bonds.
[0025] Concrete reaction process is shown in the following formula:
[0026]
[0027] (2) Weigh 33g of LHBPCS prepared in step (1) (Si-H molar content is 1.5mol) and place it in a 2L four-necked flask consisting of a stirrer, a constant pressure dropping funnel, a reflux condenser, and a nitrogen pipe. In the middle; fill the reaction system with nitrogen through the nitrogen pipe, get r...
Embodiment 2
[0034] (1) Synthesis of LHBPCS: Add 500ml THF solvent, 40.84g magnesium powder and two iodine simple substances into the reaction kettle, then add 264.84g Cl 3 SiCH 2 Cl, where magnesium and Cl 3 SiCH 2 The molar ratio of Cl is 1.05:1. After the dropwise addition, react at 60°C for 12h, then add 41.37g lithium aluminum hydride for reduction for 12h, the temperature is 60°C, after the reduction, add 68.54g, 4mol / L hydrochloric acid to quench the remaining Magnesium powder and lithium aluminum hydride, and finally filtered, pickled, washed with water, and vacuum distilled to obtain LHBPCS containing Si-H bonds.
[0035] (2) Weigh 22g of LHBPCS prepared in step (1) (Si-H molar content is 1mol) and place it in a 2L four-necked flask consisting of a stirrer, a constant pressure dropping funnel, a reflux condenser, and a nitrogen pipe. , then add 300ml THF solution to the four-necked flask, fully stir and mix; fill the reaction system with nitrogen through the nitrogen tube, and ...
Embodiment 3
[0040] (1) Synthesis of LHBPCS: Add 500ml THF solvent, 40.84g magnesium powder and two iodine simple substances into the reaction kettle, then add 264.84g Cl 3 SiCH 2 Cl, where magnesium and Cl 3 SiCH 2 The molar ratio of Cl is 1.05:1. After the dropwise addition, react at 60°C for 12h, then add 41.37g lithium aluminum hydride for reduction for 12h, the temperature is 60°C, after the reduction, add 68.54g, 4mol / L hydrochloric acid to quench the remaining Magnesium powder and lithium aluminum hydride, and finally filtered, pickled, washed with water, and vacuum distilled to obtain LHBPCS containing Si-H bonds.
[0041] (2) Weigh 22g of LHBPCS prepared in step (1) (Si-H molar content is 1mol) and place it in a 2L four-necked flask consisting of a stirrer, a constant pressure dropping funnel, a reflux condenser, and a nitrogen pipe. , and then add 300ml THF solution to the four-necked flask, fully stir and mix; fill the reaction system with nitrogen through the nitrogen tube, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com