New crystal form of pemetrexed diacid and preparation method thereof
A crystal form, dihydrogen technology, applied in the field of medicinal chemistry, can solve the problems of long preparation time, difficult separation, and difficult removal, etc., and achieves the effect of simple preparation method, small electrostatic effect, and improved quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]N-{4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pyrrole[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L - Add 10.0 g of dibenzyl glutamate to 200 ml of NaOH aqueous solution (2 mol / L), heat to 50° C., stir for 4 hours, and filter. Add 250ml of absolute ethanol to the filtrate, adjust the pH to 2-3 with dilute hydrochloric acid, and precipitate a large amount of solids. The crystallization system was heated to 55-65° C., stirred for 1 hour, cooled, filtered, and dried to obtain 6.9 g of crystal form X with a molar yield of 96%.
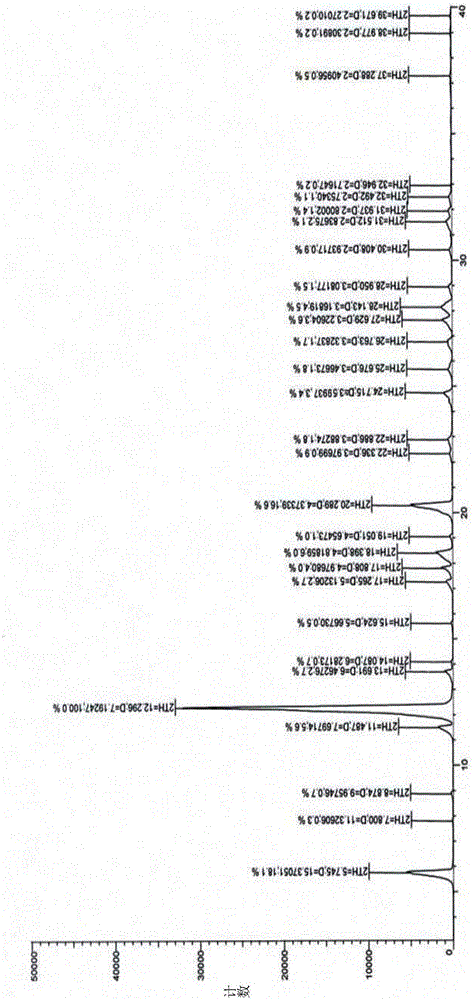
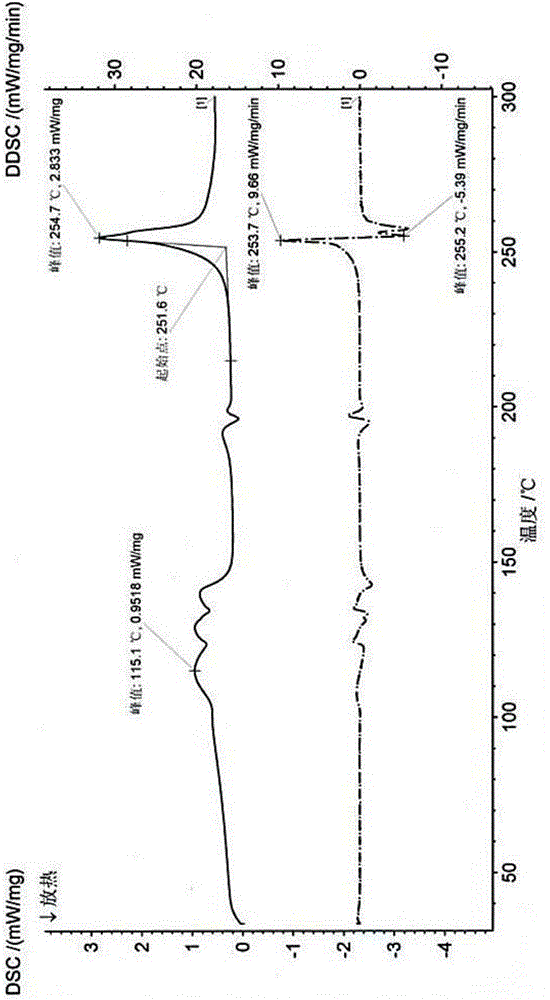
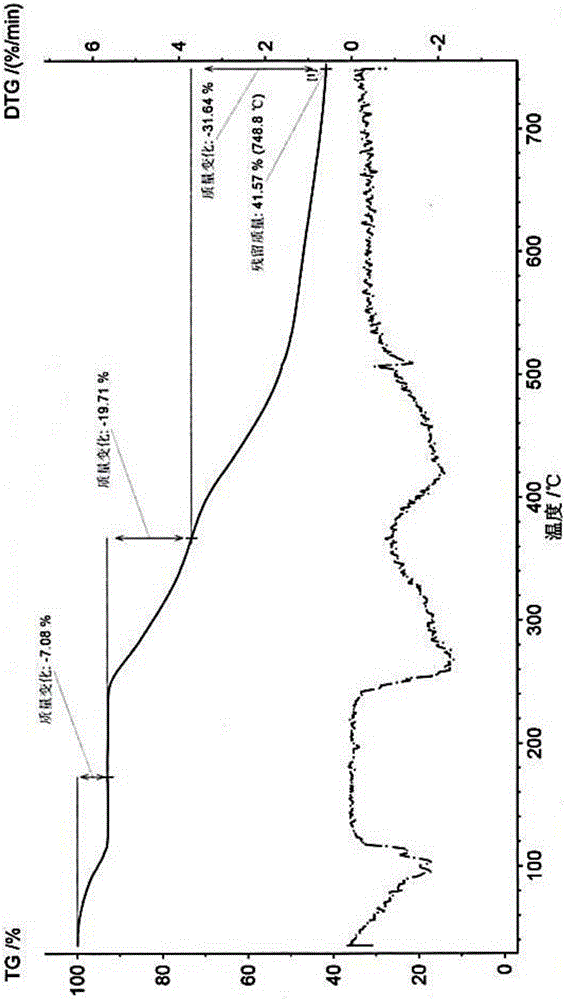
[0037] After testing and verification, its X-ray powder diffraction pattern is as follows: figure 1 As shown, its DSC spectrum, TGA spectrum and figure 2 and image 3 Matched, proving that the obtained crystal form is crystal form X.
[0038] During the process of filtering the crystals obtained in this example after being precipitated from the crystallization system, the filtrate flowed down in lines, and there was no clogging phenomenon in the whole process, ...
Embodiment 2
[0041] N-{4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pyrrole[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L - Add 10.0 g of dibenzyl glutamate to 200 ml of NaOH aqueous solution (2 mol / L), heat to 50° C., stir for 4 hours, and filter. Add 250ml of acetone to the filtrate, adjust the pH to 2-3 with dilute sulfuric acid, and precipitate a large amount of solids. The crystallization system was heated to 55-65° C., stirred for 1 hour, cooled, filtered, and dried to obtain 6.7 g of crystal form X with a molar yield of 93%.
[0042] After testing and verification, its X-ray powder diffraction pattern is as follows: figure 1 As shown, its DSC spectrum, TGA spectrum and figure 2 and image 3 Matched, proving that the obtained crystal form is crystal form X.
Embodiment 3
[0044] N-{4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pyrrole[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L - Add 10.0 g of dibenzyl glutamate to 200 ml of NaOH aqueous solution (2 mol / L), heat to 50° C., stir for 4 hours, and filter. Add 250ml of anhydrous methanol to the filtrate, adjust the pH to 2-3 with acetic acid, and precipitate a large amount of solids. The crystallization system was heated to 55-65° C., stirred for 1 hour, cooled, filtered, and dried to obtain 6.7 g of crystal form X with a molar yield of 93%.
[0045] After testing and verification, its X-ray powder diffraction pattern is as follows: figure 1 As shown, its DSC spectrum, TGA spectrum and figure 2 and image 3 Matched, proving that the obtained crystal form is crystal form X.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com