TNF-Alpha protein B cell epitopes, multiple antigen peptide with TNF-Alpha protein B cell epitopes, and application of multiple antigen peptide
A B-cell and multi-antigen technology, applied in the fields of immunology and gastroenterology, can solve the problems of difficult humoral immune response, small molecular weight, short epitope peptides, etc., and achieve the effects of low price, convenient administration and fewer times.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Screening of dominant epitopes of human TNF-α B cell epitopes and construction of multiple antigenic peptides.
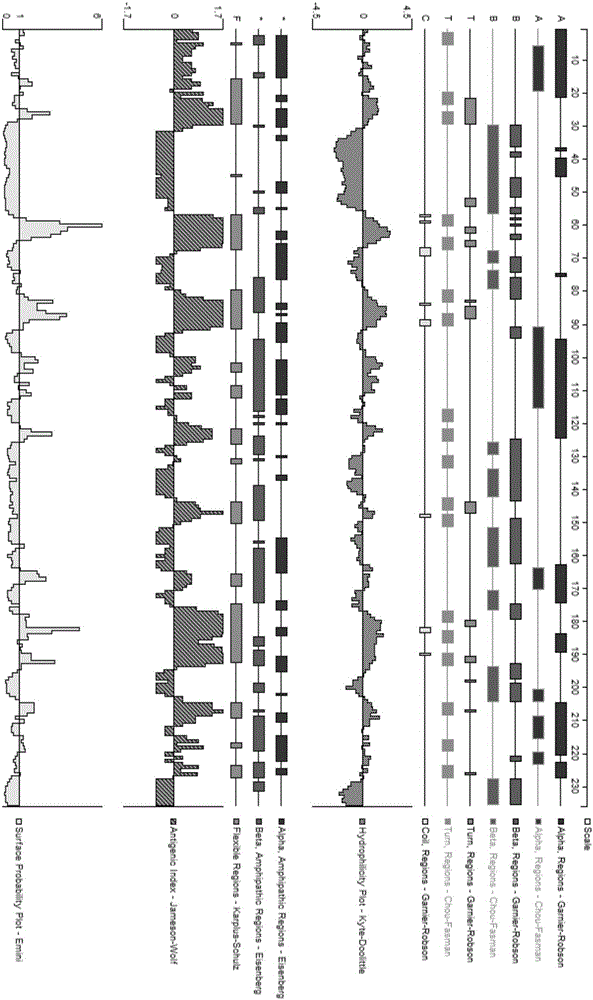
[0024] ①Search the NCBI GenBank database to obtain the amino acid sequence of human TNF-α protein; use DNAStar, GOLDENKEY (Academy of Military Medical Sciences) software and the computer vaccine design tool Bcepred on the Internet to analyze and obtain the most antigenic amino acid sequence information in the hydrophilic region and B cell epitope; according to the prediction of antigenic epitope, select a peptide rich in hydrophilic amino acids with a length of about 6-25 amino acids, while avoiding more than 4 consecutive adjacent hydrophobic amino acid residues and containing more than 2 and a half Peptides of cystine, so as not to form complex multimers through disulfide bonds.
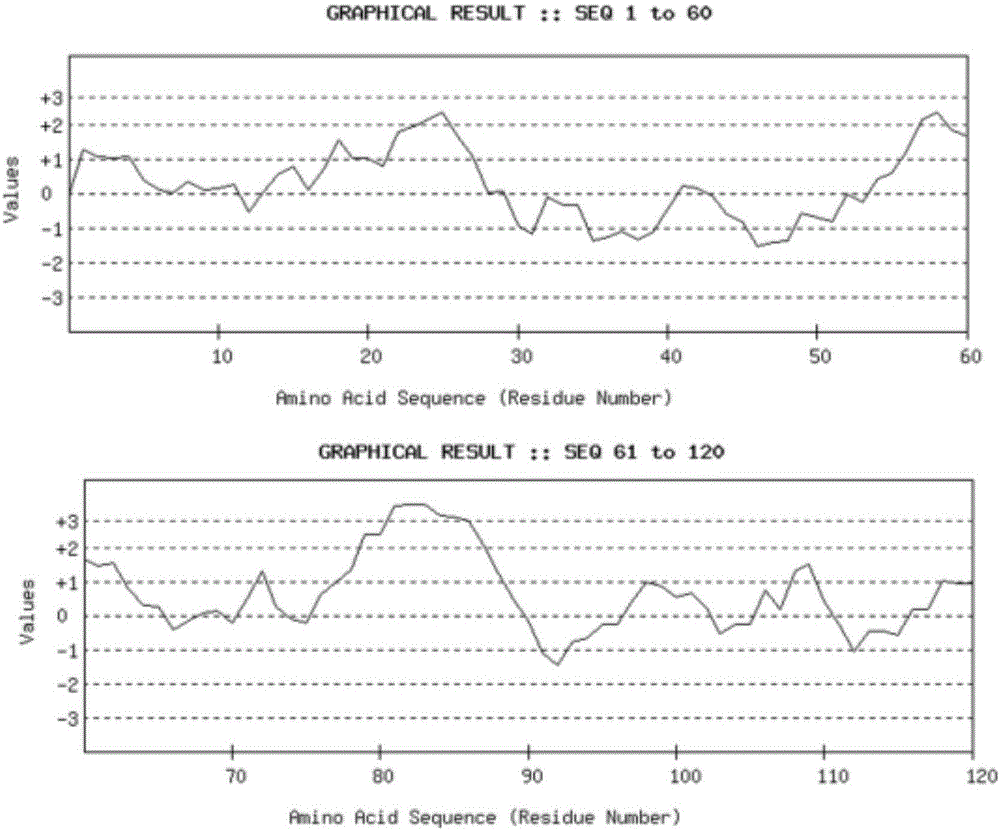
[0025] ② Analysis software ( figure 1 ) and web design tools ( figure 2 ) prediction results. Taking 13 amino acid residues as a group, analyze its hydrophilicity, ...
Embodiment 2
[0032] Example 2: Verification of the in vivo and in vitro immunological effects of the multi-antigen peptide constructed by the method described in Example 1.
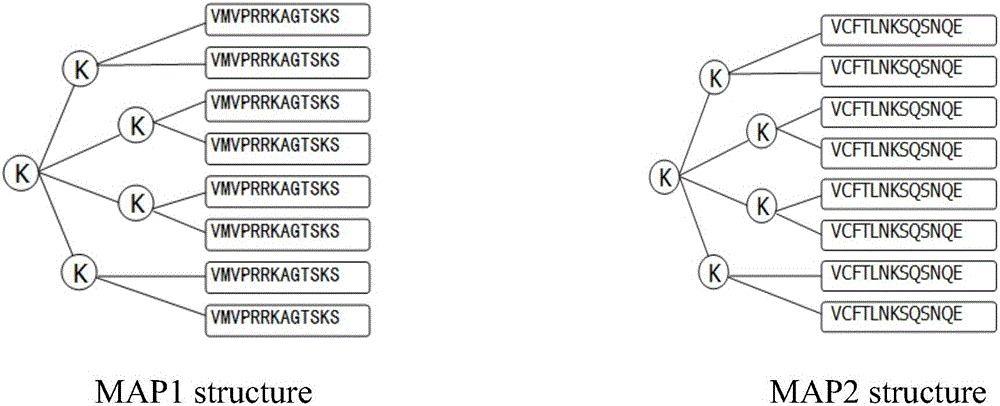
[0033] Due to the high homology between rats and human TNF-α, this embodiment takes rats as the research object, screens and identifies the dominant epitope according to the method described in Example 1, and then uses its dominant epitope to synthesize The TNF-α B cell epitope MAP multi-antigen peptide vaccine was used to verify the immunological effect of rats in vivo and in vitro, so as to prove the positive effect of the multi-antigen peptide with 8-branched peptide conformation constructed by the present invention in human and mouse ulcerative colitis.
[0034] Among them, the in vitro effect research includes: the effect of MAP antibody on the killing of mouse fibroblast L929 by TNF-α in vitro; the effect of MAP antibody on the dissolution of 3H-TdR-labeled liver cancer cell HCC97H by TNF-α in vitro. The in vivo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com