Electrochromic material based on metal nanoparticle and electrochromic device
A technology of electrochromic materials and metal nanoparticles, which is applied in the direction of color-changing fluorescent materials, instruments, chemical instruments and methods, etc., can solve the problem that electrochromic materials are difficult to obtain color changes, electrolyte materials are complicated and costly, and electrochromic colors are single and other problems, to achieve the effect of improved color change, simplified preparation, and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
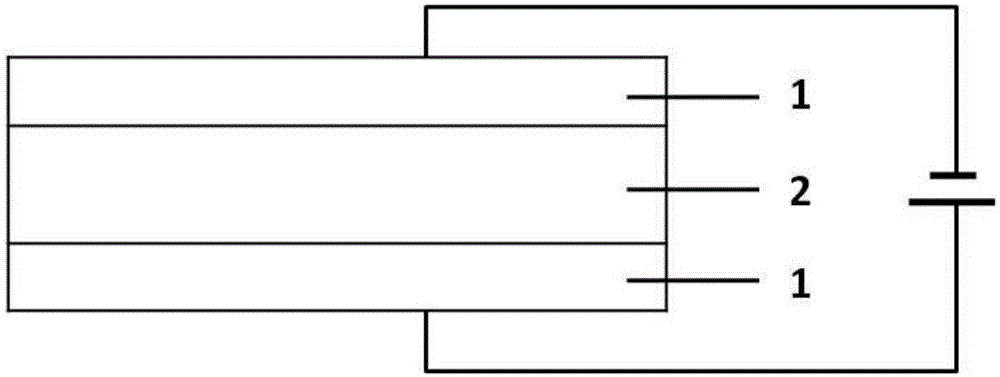
[0036] 1wt% silver acetate, 5mL acetone and 99wt% ionic liquid electrolyte 1-butyl-3-methylimidazolium bistrifluoromethanesulfonimide were rapidly stirred at room temperature to form a colorless mixed solution. Under rapid stirring, the acetone in the solution was removed under vacuum, and then H 2 As a reducing agent, silver acetate is reduced to silver nanoparticles. 0.01g of the mixed solution was injected into the device with ITO as the plate, sealed with a sealant and then tested.
[0037] Using a two-electrode system, two electrodes are added to both ends of the transparent electrode of the prepared electrochromic device, and a DC voltage of 2 V is applied to the device at intervals of 5 s with an interval of 30 s. As the power-on time increases, the device changes from the initial transparent state to yellow, red, ultraviolet and mirror states, see figure 2 . Apply reverse programming voltage to the device, and the device changes from mirror state to purple state, r...
Embodiment 2
[0039] 0.01wt% silver acetate, 5mL acetone and 89.99wt% ionic liquid electrolyte (tetrabutylammonium bistrifluoromethanesulfonimide) were stirred rapidly at room temperature to form a colorless mixed solution. Under rapid stirring, the acetone in the solution was removed under vacuum, and then H 2 Silver nitrate and palladium acetate were used as reducing agents to reduce gold and silver nanoparticles. Add 9.99wt% C6M and photoinitiator benzoin diethyl ether (IGR 651) accounting for 0.01wt% C6M addition in the synthesized gold and silver nanoparticles ionic liquid, inject 0.01g mixed solution into the device by ITO as pole plate In the test, it is sealed with a sealant and polymerized for 20 minutes under the intensity of a 365nm ultraviolet lamp of 2mW / cm2.
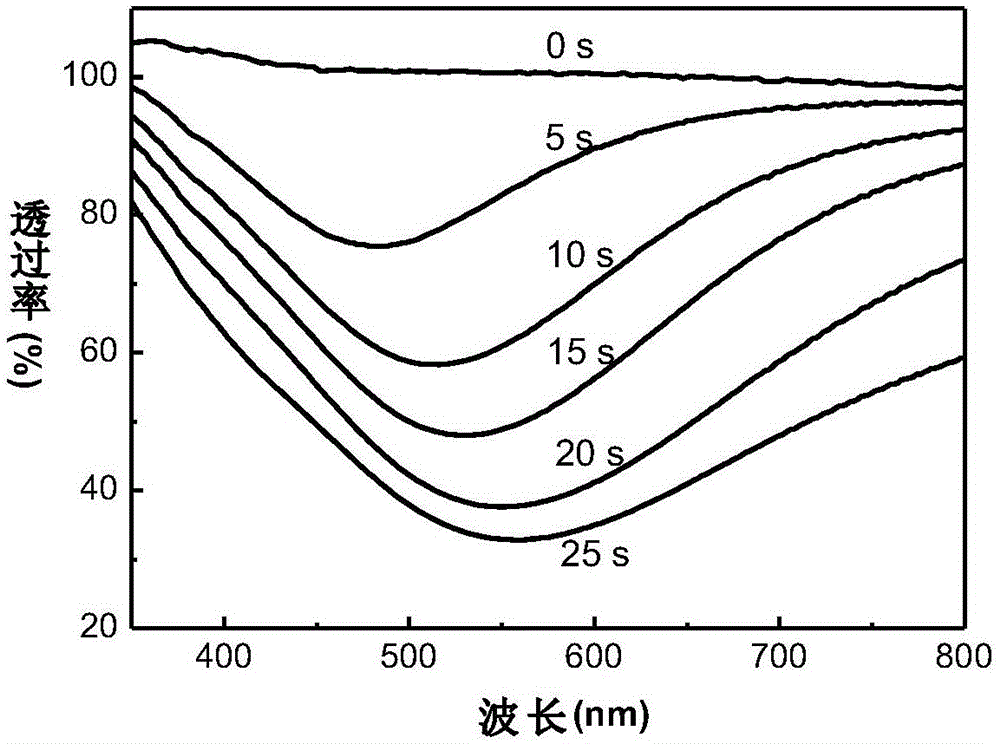
[0040] The testing method of the device is the same as that in Example 1. As the power-on time increases, the device changes from the initial transparent state to the yellow state, red state, ultraviolet state and mirr...
Embodiment 3
[0042] 1-methyl-3-(2-hydroxyethyl)imidazole bistrifluoromethanesulfonimide with 1 wt% mixed metal precursors (90 wt% silver acetate and 10 wt% palladium acetate) in 5 mL of acetone and 89.9 wt% ionic liquid electrolyte , stirred rapidly at room temperature to form a colorless mixed solution. Under rapid stirring, the acetone in the solution was removed under vacuum, and then H 2 Silver nitrate and palladium acetate are used as reducing agents to reduce silver palladium nanoparticles. Add 10 wt% glass microspheres with a diameter of 20 μm to the ionic liquid solution containing silver nanoparticles and mix evenly. 0.01g of the mixed solution was injected into the device with ITO as the plate, sealed with a sealant and then tested.
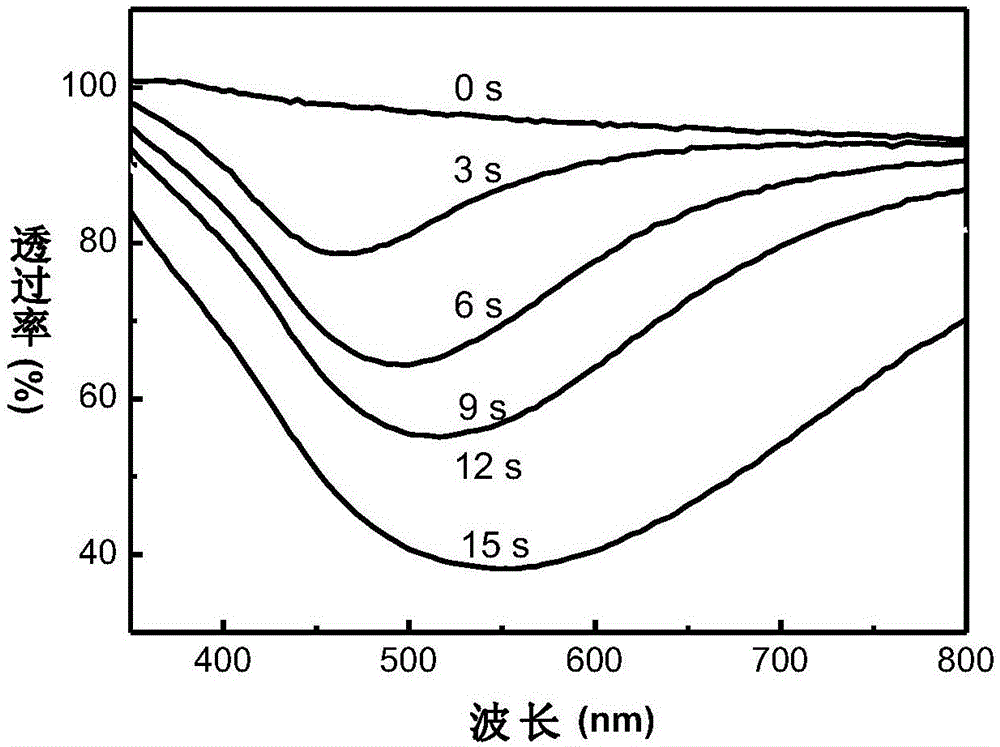
[0043] The testing method of the device is the same as that in Example 1. As the power-on time increases, the device changes from the initial transparent state to the yellow state, red state, ultraviolet state and mirror state, see Figure 4 . ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com