A kind of lithium-ion battery non-aqueous electrolyte and lithium-ion battery
A non-aqueous electrolyte and lithium-ion battery technology, applied in non-aqueous electrolyte batteries, non-aqueous electrolytes, lithium batteries, etc. Inability to work normally, etc., to achieve high charge and discharge performance, small battery deformation, and prevent deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of the lithium-ion battery of the present invention is a method for preparing lithium batteries known in the art, for example, including setting a separator between the prepared positive electrode and the negative electrode, winding or folding to form an electric core, and the electric core is accommodated in Inject the electrolyte into the battery case, and then seal the battery case to obtain a lithium-ion battery.
[0030] The non-aqueous electrolyte solution for lithium-ion batteries provided by the invention has higher high-voltage resistance performance and higher oxidation decomposition potential, and meanwhile, the battery prepared by using the non-aqueous electrolyte solution has good cycle performance and charge-discharge performance.
[0031] The lithium ion battery provided by the invention has higher energy density, first charge and discharge performance, and good storage performance and cycle performance at high temperature.
Embodiment 1
[0034] (1) Preparation of non-aqueous electrolyte:
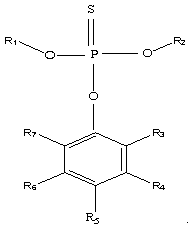
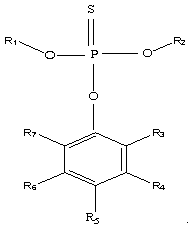
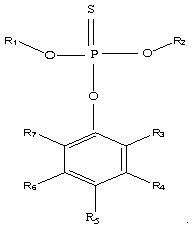
[0035] In an argon glove box, ethylene carbonate, diethyl carbonate, and dimethyl carbonate were prepared in a ratio of 2:1:3 to make 100 parts by weight of a non-aqueous solvent, and then 12 parts by weight of LiPF 6 Dissolve in the above-mentioned prepared non-aqueous solvent, then add 1 weight part of O-4-cyanophenyl O, O-dimethyl phosphorothioate (the phosphorothioate of the structure shown in the formula (1) of this application ester, where R 1 , R 2 Both are -CH 3 , R 5 -CN, R3, R4, R6, R7 are hydrogen atoms), to obtain the lithium-ion battery non-aqueous electrolyte of the present embodiment, denoted as C1;
[0036] (2) Preparation of lithium ion battery:
[0037] The positive active material (LiNi 0.5 mn 1.5 o 4 ), acetylene black, and polyvinylidene fluoride in a ratio of 90: 5: 5, and then pressed on aluminum foil to obtain a positive electrode sheet; metal lithium sheet was used as a negative electrode she...
Embodiment 2
[0039] Adopt the same steps as Example 1 to prepare non-aqueous electrolyte and button cell, the difference is: in step (1), use 1 weight part of O-(2,6-dichloro-4-tolyl)O, O-dimethyl phosphorothioate (phosphorothioate of structure shown in the application formula (1), wherein R 1 , R 2 , R 5 Both are -CH 3 , R 3 , R 7 Both are -Cl, R 4 , R 6 Both are -H) instead of O-4-cyanophenyl O, O-dimethyl phosphorothioate to prepare non-aqueous electrolyte solution C2 for lithium ion battery and button battery S2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com