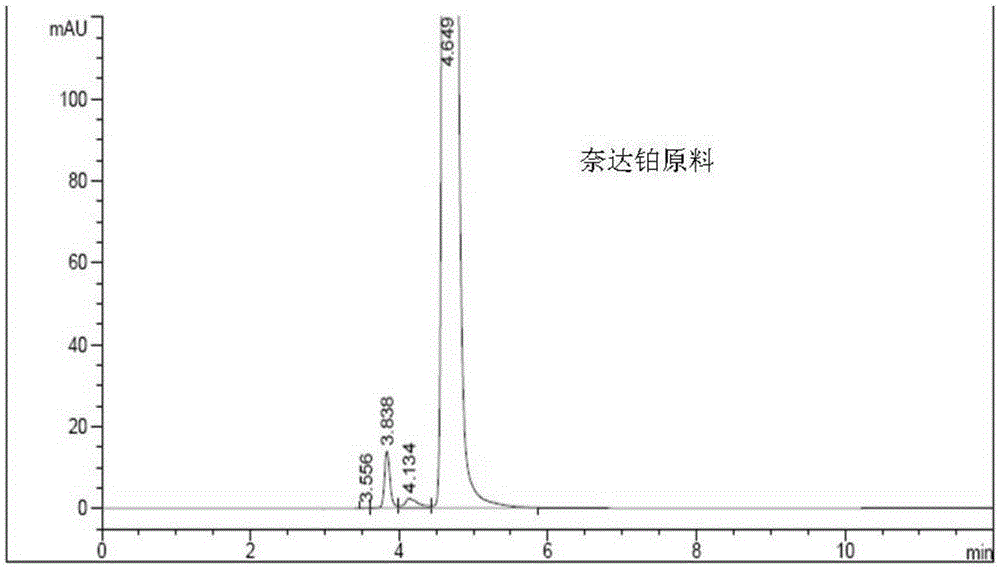
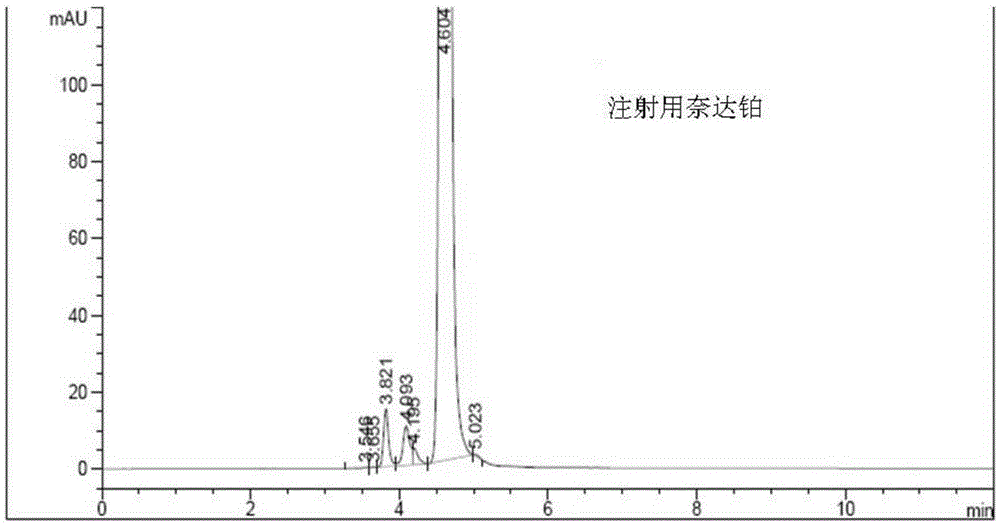
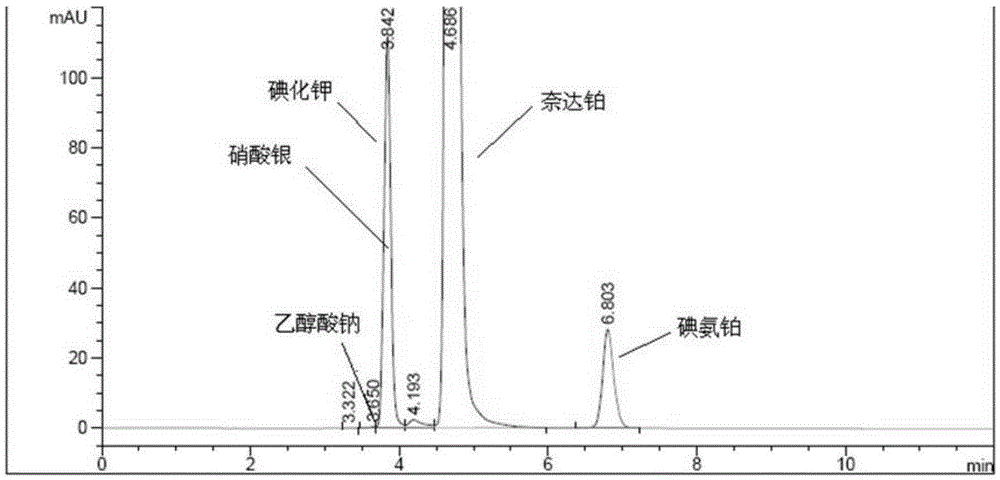
Nedaplatin impurity detection method
A detection method and technology of nedaplatin, applied in the field of HPLC analysis of nedaplatin, can solve the problems of affecting the separation of impurities, interference, affecting the accuracy of analysis, etc., to avoid potential safety hazards, improve durability and shorten the equilibration time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Chromatographic conditions:
[0036] Instrument: Agilent1200HPLC
[0037] Chromatographic column: octadecylsilane bonded silica gel column (AQ-C18, 4.6×250mm, 5μm)
[0038] Mobile phase A: 0.5% potassium dihydrogen phosphate (pH 6.5 adjusted by ammonia)
[0039] Mobile Phase B: Acetonitrile
[0040] Time (min) Mobile phase A Mobile phase B 0973 5973 157030 15.1973 25973
[0041] Column temperature: 30℃
[0042] Flow rate: 0.8ml / min
[0043] Detection wavelength: 220nm
[0044] Injection volume: 10ul
[0045] Analysis time: record the chromatogram to 3 times the retention time of the principal component peak
[0046] Solvent: mobile phase A-mobile phase B (97:3)
[0047] 2) Sample preparation:
[0048] Take an appropriate amount of raw materials and add a solvent to make a solution containing 1.0 mg of nedaplatin per ml.
[0049] Take an appropriate amount of nedaplatin for injection and add a solvent to make a solution containing 1.0mg of nedaplatin per ml.
[0050] 3) Measurement...
Embodiment 2
[0052] 1) Chromatographic conditions:
[0053] Instrument: Agilent1200HPLC
[0054] Chromatographic column: octadecylsilane bonded silica gel column (4.6×250mm, 5μm)
[0055] Mobile phase A: 1% ammonium dihydrogen phosphate (triethylamine adjusted to pH 7.5)
[0056] Mobile phase B: methanol-acetonitrile (3:2)
[0057] Time (min) Mobile phase A Mobile phase B 0982 256040 25.1982 35982
[0058] Column temperature: 25℃
[0059] Flow rate: 0.6ml / min
[0060] Detection wavelength: 220nm
[0061] Injection volume: 10ul
[0062] Analysis time: record the chromatogram to 3 times the retention time of the principal component peak
[0063] Solvent: mobile phase A-mobile phase B (98: 2)
[0064] 2) Sample preparation:
[0065] Take an appropriate amount of raw materials and add a solvent to make a solution containing 1.0 mg of nedaplatin per ml.
[0066] Take an appropriate amount of nedaplatin for injection and add a solution containing 1.0 mg of nedaplatin per ml of solvent.
[0067] 3) Measurement...
Embodiment 3
[0069] 1) Chromatographic conditions:
[0070] Instrument: Agilent1200HPLC
[0071] Chromatographic column: octadecylsilane bonded silica gel column (4.6×250mm, 5μm)
[0072] Mobile phase A: 1% ammonium dihydrogen phosphate (triethylamine adjusted to pH 7.0)
[0073] Mobile phase B: methanol
[0074] Time (min) Mobile phase A Mobile phase B 0955 5955 256040 25.1982 30955
[0075] Column temperature: 40℃
[0076] Flow rate: 1.0ml / min
[0077] Detection wavelength: 220nm
[0078] Injection volume: 20ul
[0079] Analysis time: record the chromatogram to 3 times the retention time of the principal component peak
[0080] Solvent: mobile phase A-mobile phase B (95:5)
[0081] 2) Sample preparation:
[0082] Take an appropriate amount of raw materials and add a solvent to make a solution containing 1.5 mg of nedaplatin per ml.
[0083] Take an appropriate amount of nedaplatin for injection and add a solvent to make a solution containing 1.5 mg of nedaplatin per ml.
[0084] 3) Test results: The t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com