Vaccine for prevention of respiratory syncytial virus infection
A technology for virus infection and respiratory tract, applied in the direction of antiviral agents, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve the problems of limited protection ability, limited effect, high cost, and achieve easy promotion, mature technology, low cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
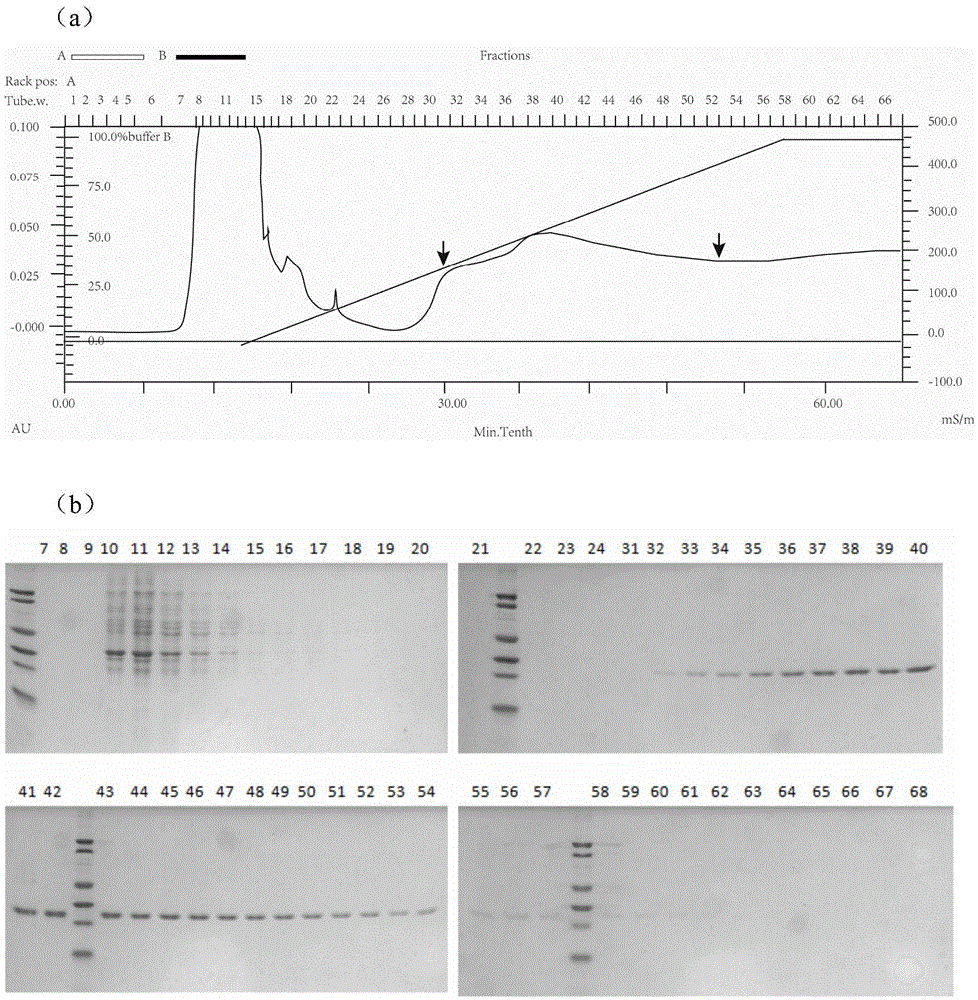
[0099] Embodiment 1, the preparation of subunit vaccine
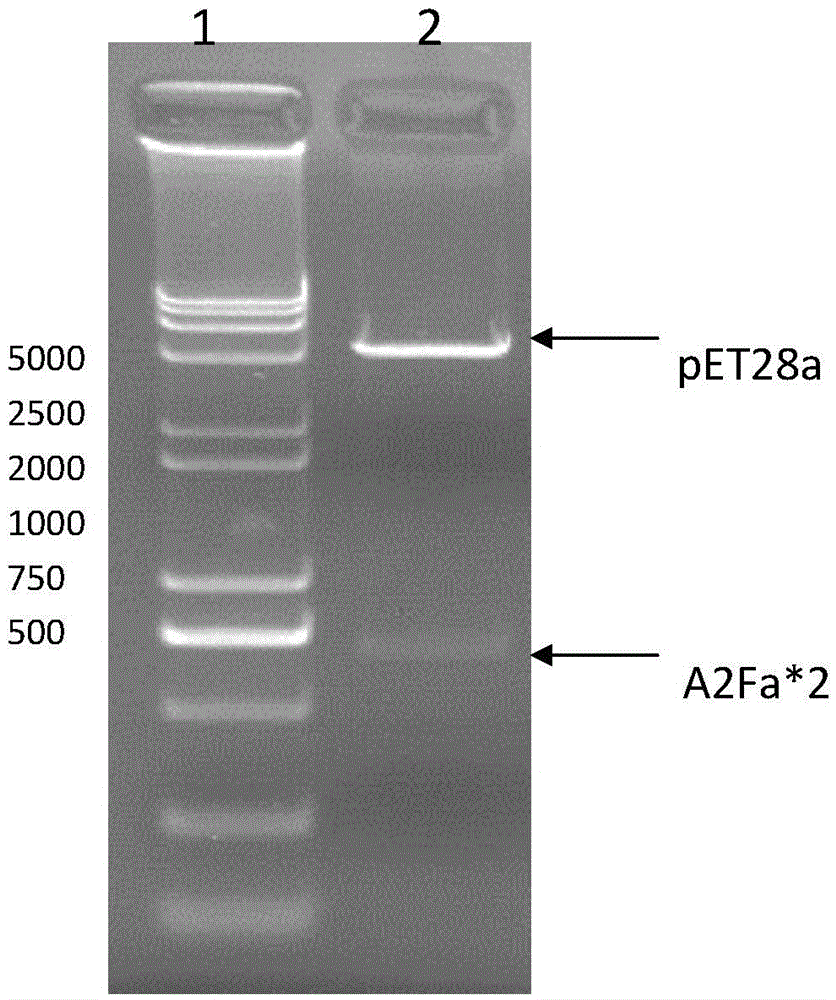
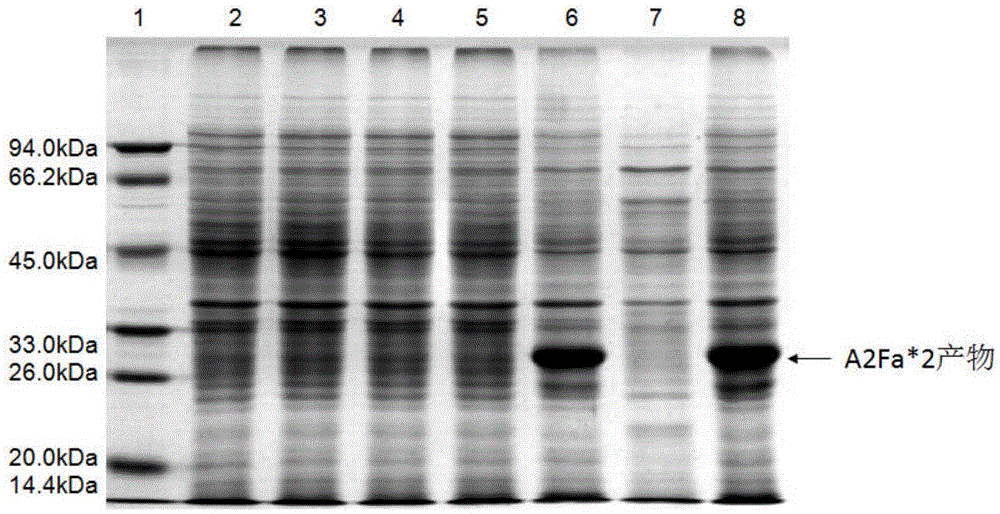
[0100] 1. Expression vector construction: RSV surface glycoprotein F was obtained by whole gene synthesis after the codon optimization of E. coli cells. The nucleotide sequence (GeneBank: AY911262.1) corresponding to the 412-524th amino acid of RSV F protein was synthesized by whole gene synthesis technology (as shown in sequence 1 in the sequence table), and repeated once, that is, 2 times of F sequence. Before synthesis, the nucleotide sequence was optimized according to the codon usage preference of Escherichia coli. The sequence optimization not only removed rare codons, but also deleted the N-terminal of the F protein, and subcloned the optimized F protein nucleotide sequence To the prokaryotic expression vector pET28a(+), named pET28a-A2Fa*2, wherein NcoI and XhoI were selected as restriction sites (as shown in sequence 2 in the sequence listing), and the size was obtained by agarose gel electrophoresis to be abo...
Embodiment 2
[0113] Example 2, Detection of Neutralizing Antibody Levels After Vaccine Immunization in Model Animals
[0114] 1. RSV F A2Fa*2 subunit vaccine immunization model animal experiments
[0115] Model animals: 6-8 weeks old female clean grade BALB / c mice were purchased from the Experimental Animal Department of Fudan University, and the experiment was divided into 5 groups with 5 mice in each group; sterile water and food were used for feeding during the experiment, and the light cycle was 12 hours ;
[0116] Vaccine and control for immunization: PBS, formalin inactivated vaccine FI-RSV (10 7 TCID 50 RSV virus (US ATCC, catalog no. VR-26 TM , reacted with formaldehyde at 37°C for 72 hours, then purified by high-speed centrifugation at 50,000g for 1 hour), and the F subunit vaccine is the above-mentioned recombinant protein;
[0117] The experimental groups and the immunization status of each group are shown in Table 2. Dissolve the F protein A2Fa*2 lyophilized product in a v...
Embodiment 3
[0129] Example 3, Detection of T lymphocyte expansion after A2Fa*2 vaccine immunization animals
[0130] 1. Experiments on A2Fa*2 subunit vaccine immunization model animals
[0131] Same as Step 1 of Example 2.
[0132] 2. Detection of T lymphocyte expansion in the cellular immune response of combined immunosuppressive RSV F protein A2Fa*2 vaccine
[0133] The mice were sacrificed on the 7th day after the last immunization in the above step 1, and the mouse spleen was taken under aseptic conditions, crushed and removed red blood cells with red blood cell lysate, filtered through a nylon column to remove B cells to make a single cell suspension (T lymphocytes) cells), washed 3 times with PBS, centrifuged and counted cells, and adjusted the cell concentration to 1×10 6 cells / ml, each group of cell suspension was added into 96-well culture plate in 3 parts, and the total number of cells in each well was 4×10 5 indivual. One part (group 1-group 7 mouse cells) plus the antigen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com