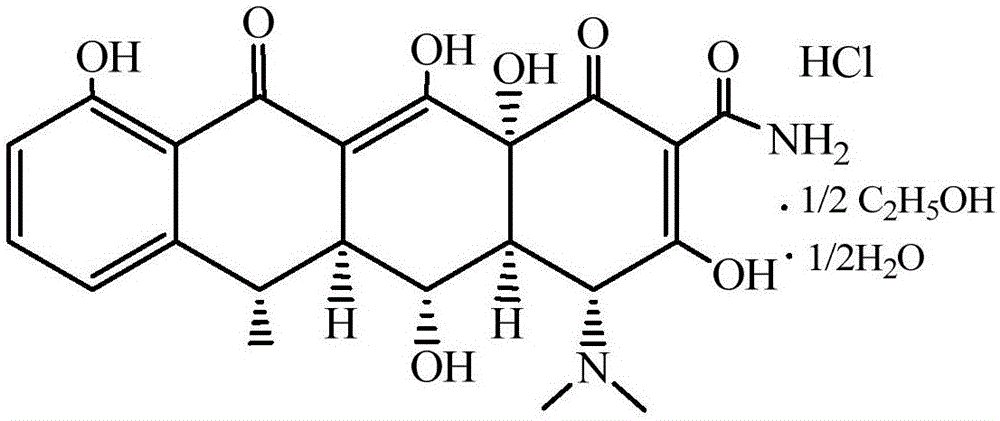
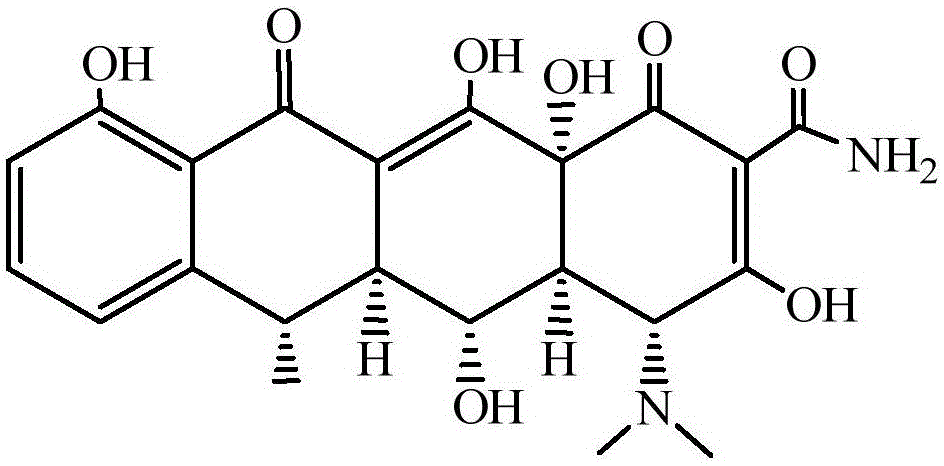
Preparation method of high-purity doxycycline hydrochloride
A technology for doxycycline hydrochloride and doxycycline, applied in the separation/purification of carboxylic acid amide, organic chemistry, etc., can solve the problems of reduced yield, increased solvent usage and residue, etc., to achieve production The effect of low cost, short process time and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 6-methyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-1,11-dioxo-l,4,4a,5,5a,6,11,12a- The preparation method of octahydro-2-tetracenecarboxamide (doxycycline) (attached figure 2 )
[0026] Weigh 100g doxycycline hydrochloride raw material, add in the reactor, then add purified water 500ml, after stirring and dissolving for 90 minutes, the doxycycline hydrochloride aqueous solution is filtered with a filter paper with a pore size of 1 μm, and the solid insoluble impurities are filtered out to obtain doxycycline hydrochloride in water. Add the filtered doxycycline hydrochloride aqueous solution into the reactor, and adjust the temperature to 20°C. 2.5% ammonia water was added dropwise to the reactor at a rate of 3 ml / min to adjust the pH value to 3.0, the reaction crystallized, and a solid was precipitated, which was filtered and dried to obtain 84.5 g of doxycycline free base crystals.
[0027] 6-methyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-1,11-dioxo-l,4,4a,...
Embodiment 2
[0030] 6-methyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-1,11-dioxo-l,4,4a,5,5a,6,11,12a- The preparation method of octahydro-2-tetracenecarboxamide (doxycycline) (attached figure 2 )
[0031] Weigh 100g doxycycline hydrochloride raw material, add in the reactor, then add purified water 2000ml, after stirring and dissolving for 30 minutes, the doxycycline hydrochloride aqueous solution is filtered with a filter paper with a pore size of 30 μm, and the solid insoluble impurities are filtered out to obtain doxycycline hydrochloride in water. Add the filtered doxycycline hydrochloride aqueous solution into the reactor, and adjust the temperature to 60°C. Sodium bicarbonate was added dropwise to the reactor at a rate of 5 ml / min to adjust the pH value to 2.1, the reaction crystallized, and a solid was precipitated, which was filtered and dried to obtain 82.9 g of doxycycline free base crystals.
[0032] 6-methyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-1,11-dioxo-l,4,4...
Embodiment 3
[0035] 6-methyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-1,11-dioxo-l,4,4a,5,5a,6,11,12a- The preparation method of octahydro-2-tetracenecarboxamide (doxycycline) (attached figure 2 )
[0036] Weigh 100g doxycycline hydrochloride raw material, add in the reactor, then add purified water 1000ml, after stirring and dissolving for 10 minutes, the doxycycline hydrochloride aqueous solution is filtered with a filter paper with a pore size of 50 μm, and solid insoluble impurities are filtered out to obtain doxycycline hydrochloride in water. Add the filtered doxycycline hydrochloride aqueous solution into the reactor, and adjust the temperature to 50°C. Add sodium hydroxide solution dropwise to the reactor at a rate of 10 ml / min, adjust the pH value to 1.8, react to crystallize, and precipitate a solid, filter, and dry to obtain 83.1 g of doxycycline free base crystals.
[0037] 6-methyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-1,11-dioxo-l,4,4a,5,5a,6,11,12a- Octahydro-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com