Method for preparing high-purity Gadobutrol
A high-purity technology of gadobutrol, which is applied in the field of preparation of high-purity gadobutrol, can solve the difficulties of intermediates, product yield and purity decline, inability to reproduce gadobutrol purity and yield, purification and quality control to achieve the effects of easy industrial application, high purity and yield, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
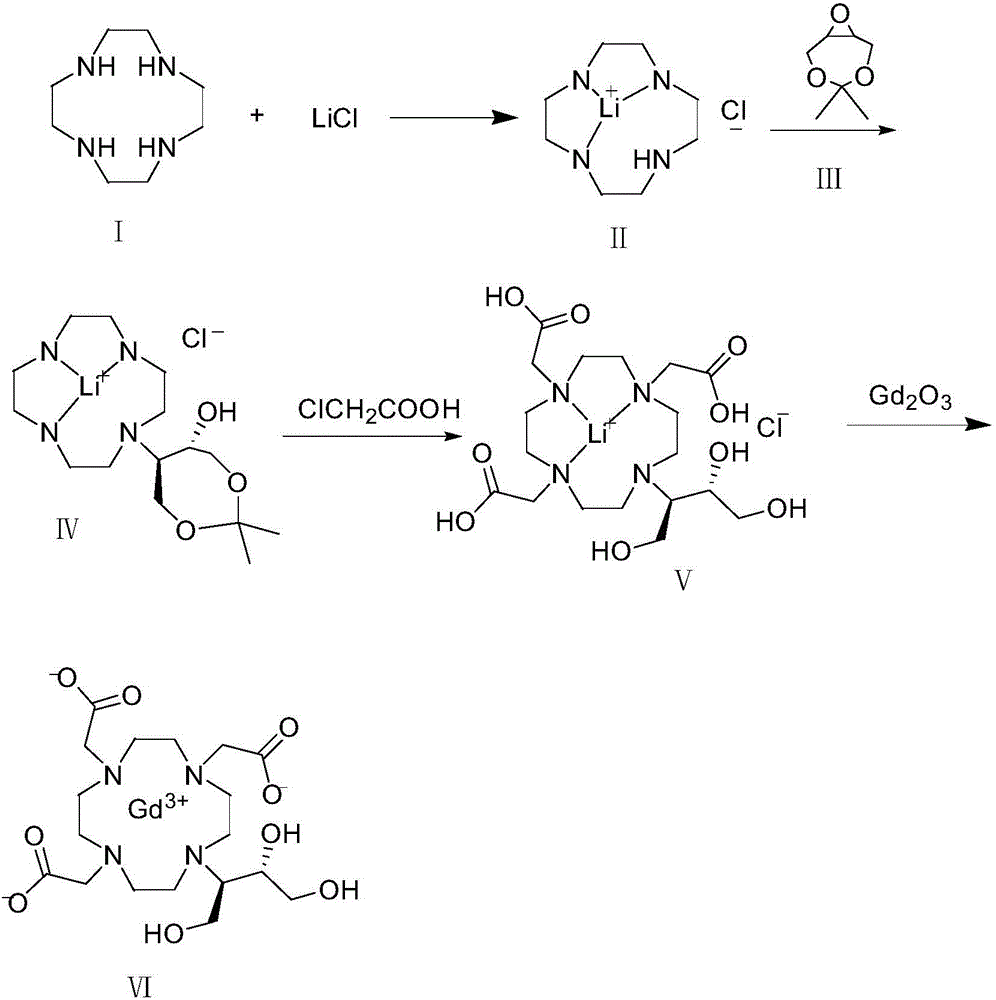
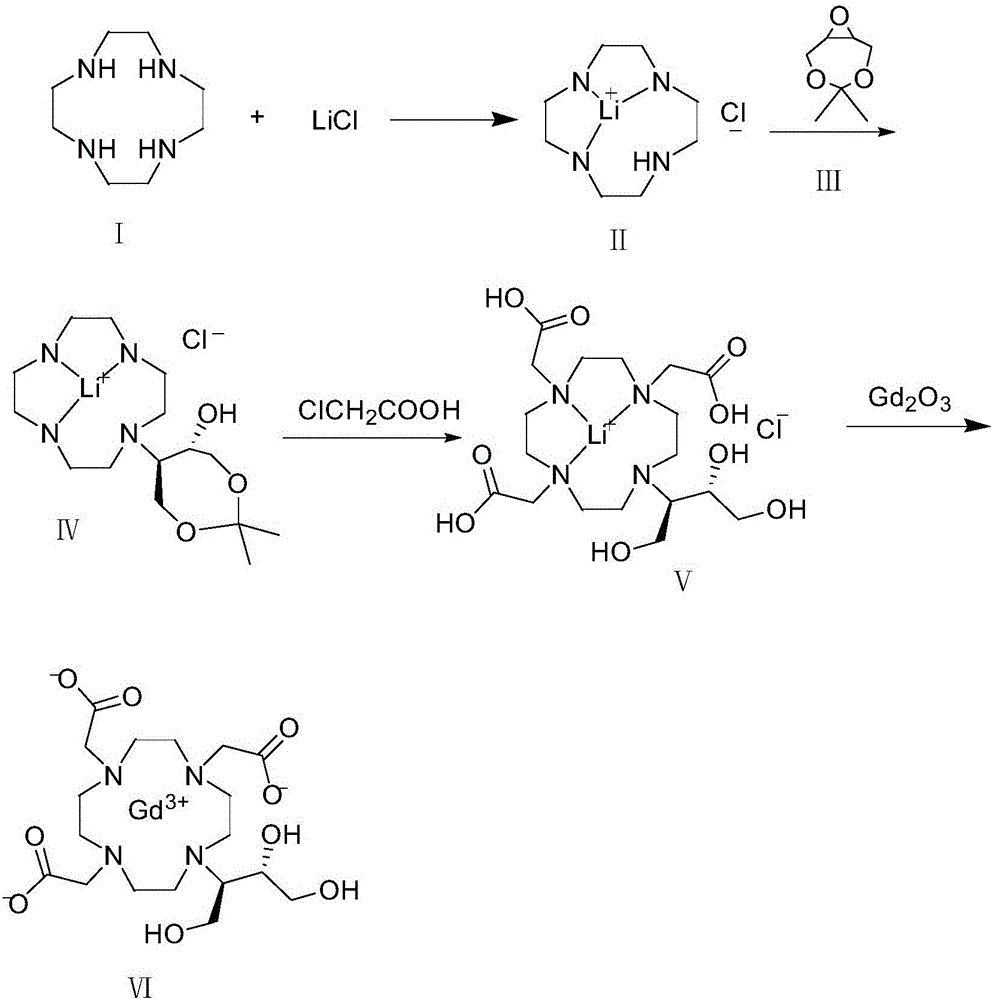
[0036] Take 5.0 g of Lunhuan Tengning (I) and 1.85 g of lithium chloride in a three-necked flask, add 30 ml of isopropanol, heat up to 80-85°C and reflux for 24 hours, then concentrate to dryness under reduced pressure. Add 30ml of anhydrous THF to the concentrated solution, stir to dissolve, add 5.0g of 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]octane (II), heat up and reflux for 5 hours , concentrated to dryness under reduced pressure, added methyl tert-butyl ether to reflux and crystallized, filtered and dried under vacuum at 50°C to obtain 8.1 g of intermediate (IV).
[0037] Add 8.0 g of intermediate (IV) to 8 ml of purified water and stir to dissolve. Take another 7.37g of chloroacetic acid dissolved in 8ml of purified water, add 3.27g of lithium hydroxide monohydrate at -5-5°C, stir to dissolve, add the lithium chloroacetate solution dropwise to the intermediate (IV) solution at room temperature, and the dropwise addition ends Raise the temperature to 65°C, add about 2.8g...
Embodiment 2
[0041] Take 32g of Lunhuan Tengning (I) and 11.8g of lithium chloride in a three-necked flask, add 200ml of isopropanol, heat up to 80-85°C and reflux for 24h, then concentrate to dryness under reduced pressure. Add 200ml of anhydrous THF to the concentrated solution, stir to dissolve, add 32.1g of 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]octane (II), and heat up to reflux for 5 hours , concentrated to dryness under reduced pressure, added methyl tert-butyl ether to reflux and crystallized, filtered and dried under vacuum at 50°C to obtain 56g of intermediate (IV).
[0042] Add 56ml of purified water to 56g of intermediate (IV) and stir to dissolve. Take another 51.6g of chloroacetic acid dissolved in 52ml of purified water, add 22.9g of lithium hydroxide monohydrate at -5-5°C, stir to dissolve, add the lithium chloroacetate solution dropwise to the intermediate (IV) solution at room temperature, and the dropwise addition ends Raise the temperature to 65°C, add about 20g of lit...
Embodiment 3
[0046] Take 128g of Lunhuan Tengning (I) and 47.2g of lithium chloride in a three-necked flask, add 800ml of isopropanol, heat up to 80-85°C and reflux for 24h, then concentrate to dryness under reduced pressure. Add 800ml of anhydrous THF to the concentrated solution, stir to dissolve, add 128.5g of 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]octane (II), and heat up to reflux for 5 hours , concentrated to dryness under reduced pressure, added methyl tert-butyl ether to reflux and crystallized, filtered and dried under vacuum at 50°C to obtain 240 g of intermediate (IV).
[0047] Add 240ml of purified water to 240g of intermediate (IV) and stir to dissolve. Another 221g of chloroacetic acid was dissolved in 221ml of purified water, and 98.2g of lithium hydroxide monohydrate was added at -5-5°C, stirred to dissolve, and the lithium chloroacetate solution was added dropwise to the intermediate (IV) solution at room temperature, and the temperature was raised at the end of the dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com