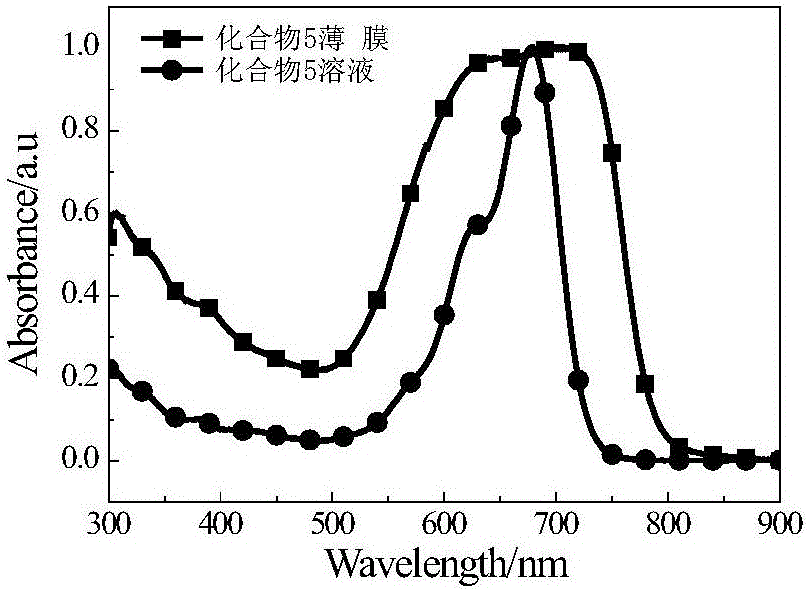
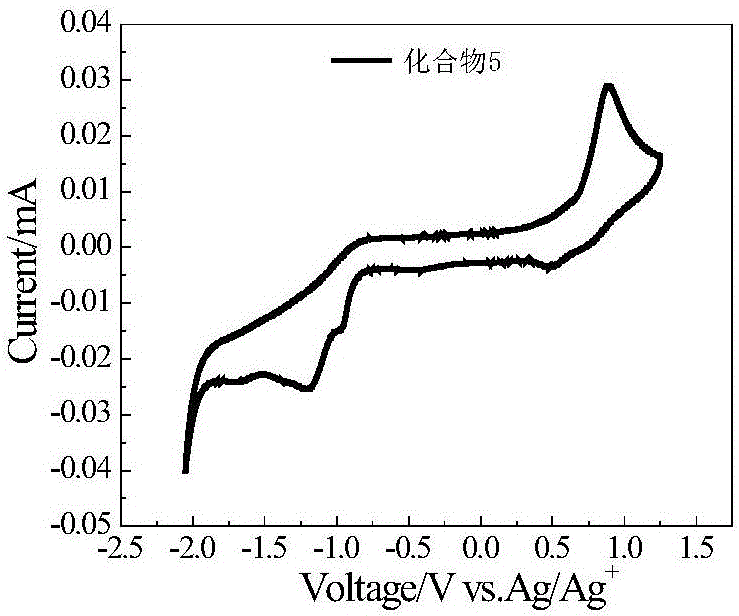
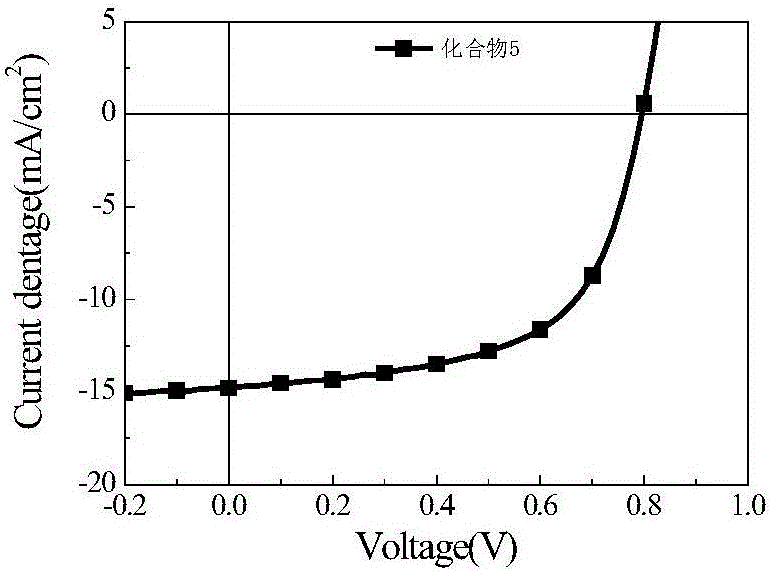
A-D-A type conjugated molecules based on substituted indenothiophene condensed ring unit and preparation method thereof
A conjugated molecule, A-D-A technology, used in electrical components, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., can solve the problems of insufficiency of devices, low photoelectric conversion efficiency, etc., and achieve a wide absorption wavelength range, high photoelectric conversion efficiency, good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of compound 5:
[0033] The synthetic route is as follows:
[0034]
[0035] Synthetic route of Scheme.1 compound 5
[0036] (1) Synthesis of Compound 3:
[0037] Add 9.3g of 4-bromo-phenylhexyl ether under the protection of nitrogen, dissolve it in 60ml of dry tetrahydrofuran, drop the temperature to -78°C, add 18ml of 2.0M butyl lithium dropwise, keep stirring at low temperature for 1 hour after dropping, and then add 3g of compound a dropwise 60ml of tetrahydrofuran solution, keep stirring at -78°C for 1 hour after dripping, monitor by TLC, add water to stop the reaction after the reaction is complete, extract, wash, spin off the solvent, the product obtained is recrystallized with ethanol, and then the light yellow solid is directly added to Glacial acetic acid 50ml and H 2 SO 4 Stir in 1ml of the system for 4h to terminate the reaction and recrystallize to obtain 4.5g of compound 3 with a yield of 70%. 1 H NMR (500MHz, CDCl 3 ),...
Embodiment 2
[0044] Embodiment 2: the synthesis of compound 9
[0045] The synthetic route is as follows:
[0046]
[0047] Synthetic route of Scheme.2 compound 9
[0048] (1) Synthesis of Compound 7
[0049] Add 9.3g of 3-bromo-phenylhexyl ether under the protection of nitrogen, dissolve it in dry tetrahydrofuran, drop the temperature to -78°C, add 18ml of 2.0M butyl lithium dropwise, stir for 1 hour after dropping, then add dropwise 3g of compound a in tetrahydrofuran 60ml, keep stirring at -78°C for 1 hour after dripping, monitor by TLC, add water to stop the reaction after the reaction is complete, extract, wash, and spin off the solvent. The product obtained is recrystallized with ethanol, and then the beige solid is directly added to 50ml of glacial acetic acid and H 2 SO 4 Stir in 1ml of the system for 4h, terminate the reaction and recrystallize to obtain 4.1g of compound 7 with a yield of 63%. 1 H NMR (500MHz, CDCl 3 ),7.51(s,2H),7.25(s,2H),7.16(t,J=10Hz,4H),6.84(m,8H),6...
Embodiment 3
[0056] Embodiment 3: the synthesis of compound 13
[0057] The synthetic route is as follows:
[0058]
[0059] Synthetic route of Scheme.3 compound 13
[0060] (1) Synthesis of Compound 11:
[0061] Add 9.9g of 2-fluoro-4-bromo-phenylhexyl ether under the protection of nitrogen, dissolve in 60ml of dry tetrahydrofuran, drop the temperature to -78°C, add 18ml of 2.0M butyllithium dropwise, stir for 1 hour after dropping, and then dropwise add 2 , Ethyl 5-bis(2-thiophene[3,2-b]thienyl)phthalate 3g / tetrahydrofuran 60ml, keep stirring at -78°C for 1 hour after dropping, monitor by TLC, stop the reaction after the reaction is complete, Extraction, washing, drying, concentration of solvent, recrystallization and suction filtration to obtain solid product, directly add glacial acetic acid 50ml and H 2 SO 4 1 ml was stirred for 4 hours to terminate the reaction, and recrystallized after suction filtration and washing to obtain 4.5 g of product 11 with a yield of 65%. 1 H NMR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com