Method for preparing methyl cyclohexanecarboxylate from methyl benzoate through hydrogenation reaction
A technology of methyl benzoate and cyclohexanecarboxylic acid, which is applied in the field of producing methyl cyclohexanecarboxylate through the hydrogenation reaction of methyl benzoate, can solve the problems of catalyst loss, achieve reduced production costs, good loss resistance, and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Catalyst preparation:
[0022] After the activated carbon carrier was ground and sieved to 100 meshes, it was placed in an oven at 120°C and dried overnight; an equal volume impregnation method was used to prepare a 1.5 mg / ml ruthenium trichloride precursor impregnating solution equivalent to the saturated water absorption of the carrier, and stirred The carrier was added to make it evenly mixed. After ultrasonic treatment, it was left to stand for 24 hours, and then dried overnight in an oven at 110°C. The loading amount of Ru is 5 wt.%.
Embodiment 2
[0023] The preparation of embodiment 2 methyl benzoate:
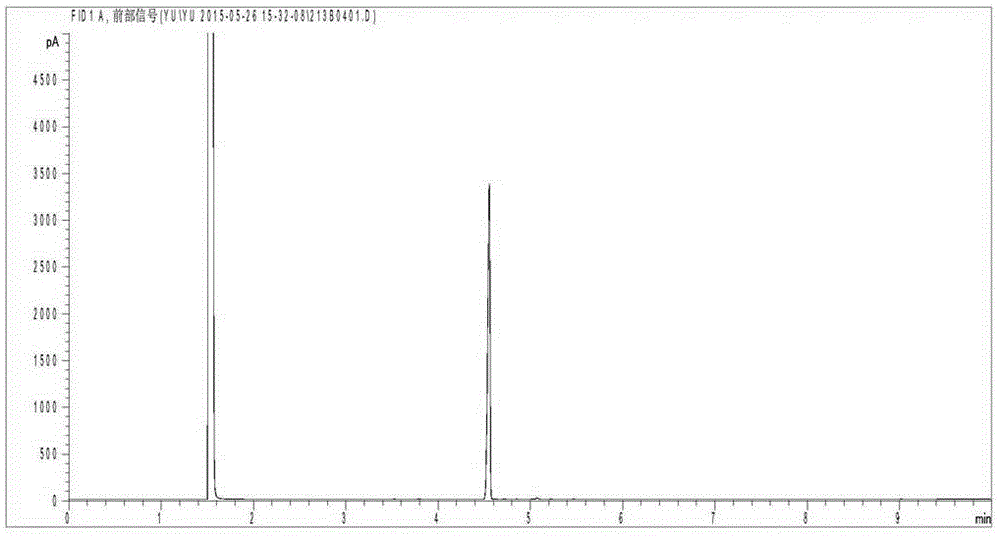
[0024] 0.25 g of the Ru / C catalyst prepared in Example 1 was weighed and placed in a tube furnace for hydrogen reduction at a reduction temperature of 350° C. and a reduction time of 3 h. Add the reduced catalyst, 4g of methyl benzoate, and 20ml of methanol into the reaction kettle, and stir to disperse evenly. Seal the reactor, replace the air with hydrogen gas and heat it to 120°C, then flush with 3MPa hydrogen gas and keep it warm for 3h. After the reaction, the reaction kettle was taken out, cooled to room temperature, opened after the pressure was reduced, and the liquid product was taken out and analyzed by chromatography. Analysis results showed that the conversion rate of methyl benzoate was 65%, and the product selectivity was 94%.
Embodiment 3
[0026] According to the method in Example 1, the activated carbon carrier is replaced by SiO 2 Support, preparation of Ru / SiO 2 . Then weigh 0.25g of the prepared Ru / SiO 2 The catalyst was reduced with hydrogen, the reduction temperature was 400°C, and the reduction time was 3h. Add the reduced catalyst, 4g of methyl benzoate, and 30ml of methanol into the reaction kettle, and stir to disperse evenly. Seal the reactor, replace the air with hydrogen gas and heat it to 120°C, then flush with 3MPa hydrogen gas and keep it warm for 2h. After the reaction, the reaction kettle was taken out, cooled to room temperature, opened after the pressure was reduced, and the liquid product was taken out and analyzed by chromatography. Analysis results show that the conversion rate of methyl benzoate is 96%, and the product selectivity is >95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com