A kind of preparation method of oxalum iodide
A technology of oxalidium and phenyl, applied in the field of preparation of oxalidium, can solve the problems of being unable to adapt to industrialized production, having great harm to human body, being difficult to obtain, etc., and achieving short process reaction time, convenient operation and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
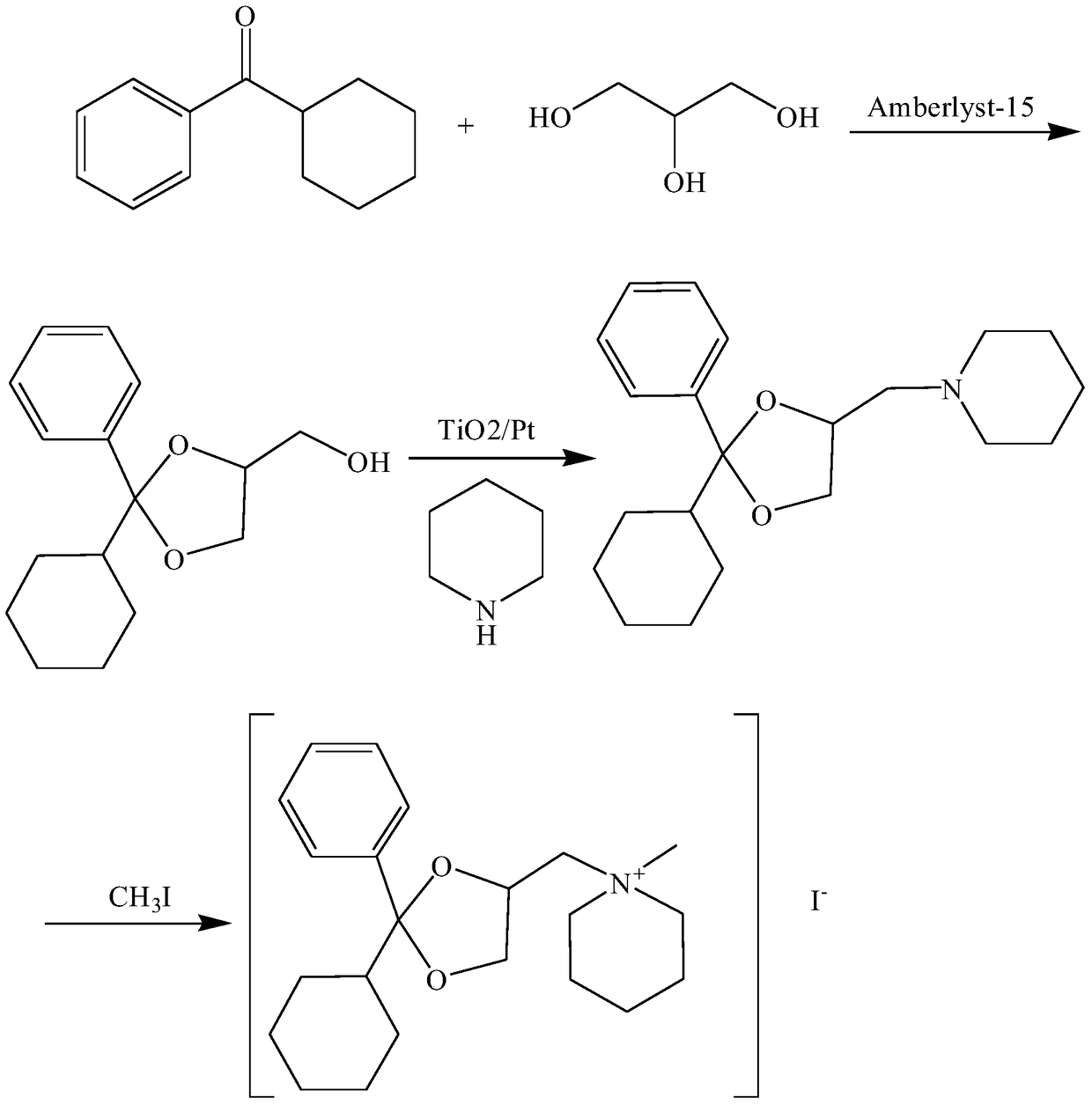
[0025] refer to figure 1 , the preparation method of a kind of oxalamium iodide that the present invention proposes, comprises the steps:
[0026] S1. Preparation of 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxol: mix cyclohexyl phenyl ketone, glycerin, Amberlyst-15 exchange resin, and color-changing silica gel, heat up, and keep stirring , filter, take the filtrate and add saturated aqueous sodium bicarbonate solution to wash, let the organic layer stand still, dry, and distill under reduced pressure to obtain material A; purify material A and dry to obtain 2-phenyl-2-cyclohexyl-4-methanol-1 ,3-dioxin;
[0027] S2. Preparation of 2-phenyl-2-cyclohexyl-4-piperidinylmethyl-1,3-dioxol: take piperidine, pass through an inert gas, and sequentially add 2-phenyl-2- Cyclohexyl-4-methanol-1,3-dioxol, catalyst TiO 2 / Pt mixing, adjust the temperature, irradiate with a high-pressure mercury lamp, stir continuously during the irradiation process, filter, take the filtrate and concentrate...
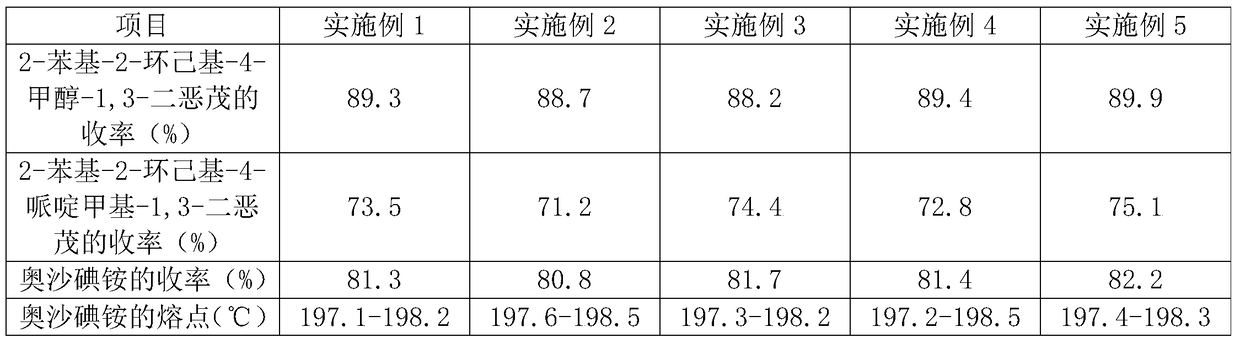
Embodiment 1
[0031] A preparation method for oxalum iodide, comprising the steps of:
[0032] S1. Preparation of 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxol: 19 parts by weight of cyclohexyl phenyl ketone, 15 parts of glycerin, 0.18 parts of Amberlyst-15 exchange resin, 0.12 Mix color-changing silica gel, heat up to 80°C, heat and stir for 90 minutes, filter, take the filtrate and add saturated sodium bicarbonate aqueous solution to wash, let the organic layer stand still, dry, and distill under reduced pressure to obtain material A; recrystallize material A with ethanol, Dry to obtain 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxol;
[0033] S2. Preparation of 2-phenyl-2-cyclohexyl-4-piperidinylmethyl-1,3-dioxol: take 34 parts of piperidine in parts by weight, pass through argon, and add 52 parts obtained in S1 in sequence 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxol, 1 part catalyst TiO 2 / Pt mix, adjust the temperature to 30°C, irradiate with a 400w high-pressure mercury lamp for 24 hours,...
Embodiment 2
[0036] A preparation method for oxalum iodide, comprising the steps of:
[0037]S1. Preparation of 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxol: 21 parts by weight of cyclohexyl phenyl ketone, 13 parts of glycerin, 0.3 parts of Amberlyst-15 exchange resin, Mix evenly with 0.08 color-changing silica gel, heat up to 90°C, keep stirring for 60 minutes, filter, take the filtrate and add saturated sodium bicarbonate aqueous solution to wash, let the organic layer stand still, dry, and distill under reduced pressure to obtain material A; recrystallize material A with acetonitrile, Dry to obtain 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxol;
[0038] S2. Preparation of 2-phenyl-2-cyclohexyl-4-piperidinylmethyl-1,3-dioxol: take 51 parts of piperidine in parts by weight, pass through argon, and add 26 parts obtained in S1 in sequence 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxol, 2 parts catalyst TiO 2 / Pt mix well, adjust the temperature to 10°C, irradiate with a 500w high-pressure mercu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com