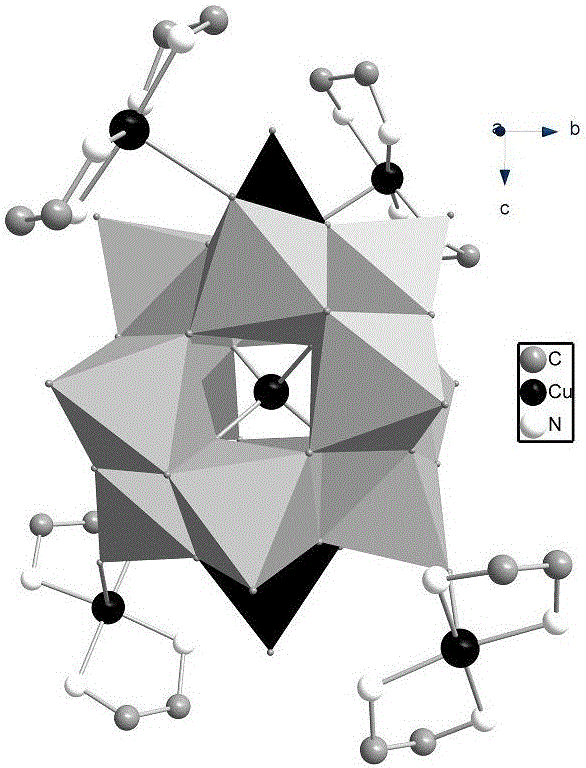
Heteropolycopper niobate, preparation method thereof and application thereof in photocatalytic degradation of dye wastewater
A technology for dye wastewater and niobate, which is applied in the synthesis of polyniobate and the field of photocatalysis, and achieves the effects of good application prospect, low cost and simple synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Heteropolycopper Niobates
[0025] 1. Precursor K 8 [Nb 6 o 19 ]•17H 2 O was prepared according to the method reported in the literature (J. Am. Chem. Soc., 2012, 19716–19721);
[0026] 2. Stir and mix 5mL high-purity water and 10mL ethylene glycol to form a mixed solvent, then add 0.5mL ethylenediamine, 0.1g copper sulfate pentahydrate, and 0.3g precursor K to the mixed solvent in sequence 8 [Nb 6 o 19 ]•17H 2 0 and 0.2g sodium metavanadate, stir and mix to obtain mixed solution;
[0027] 3. Transfer the mixed solution to a hydrothermal reaction kettle, then place the hydrothermal reaction kettle in a programmable temperature-controlled oven, set the temperature parameters of the oven, raise the temperature from room temperature to 110°C in 1 hour, and keep the temperature at 110°C for 72 hours , and then lowered the temperature to 30°C in 48h, and finally obtained the target product dark purple massive crystal heteropolycopper niobate with a yie...
Embodiment 2
[0034] Photocatalytic degradation activity test
[0035] Add 50mL rhodamine B solution of a certain concentration to the quartz photocatalytic reaction tube, then add a certain quality of heteropolycopper niobate, adjust the pH value of the reaction system through dilute sulfuric acid and potassium hydroxide solution, and then start the photocatalytic reaction from the quartz A constant flow of air was introduced into the bottom of the tube, irradiated with a 300W mercury lamp for a certain period of time, 5mL of the sample liquid was taken, and after it was centrifuged, the supernatant liquid was taken, and the absorbance of the sample liquid at 554nm was measured with a UV-Vis spectrophotometer. Since the concentration of Rhodamine B has a linear relationship with its absorbance, the degradation rate of Rhodamine B can be calculated from the absorbance.
[0036] The effect of the amount of heteropolycopper niobate on the photocatalytic effect:
[0037] Adding 50mL mass conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com