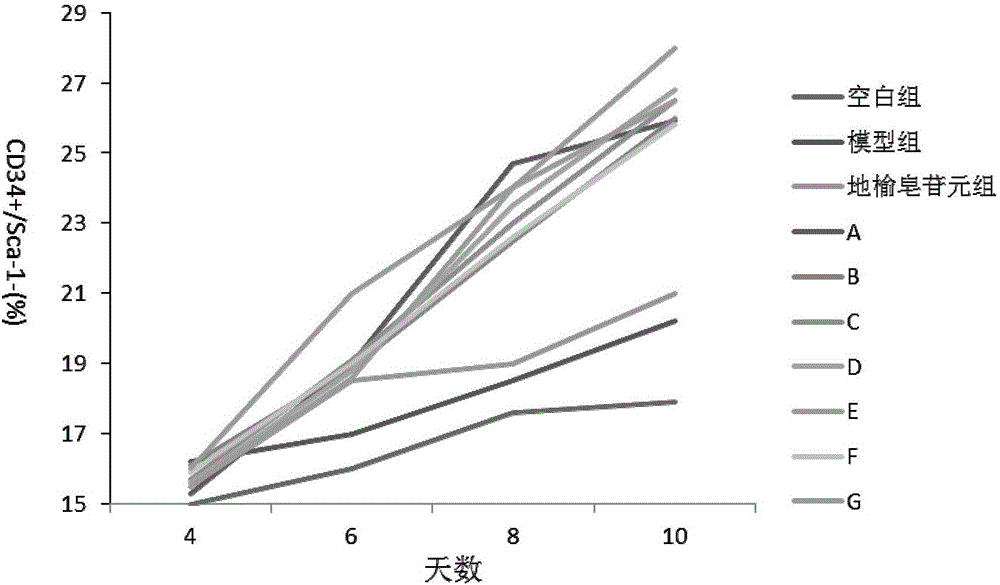
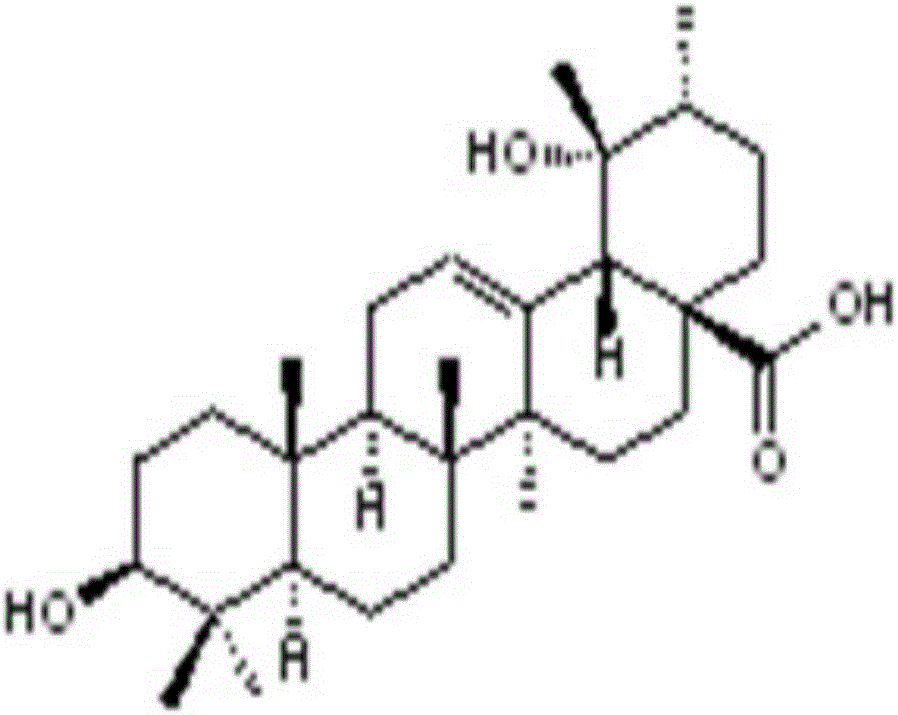
Sanguisorba officinalis aglycone lipidosome, and preparation method and purpose thereof
A technology of elmia aglycone lipid and fructus aglycone, which is applied in the field of medicine and can solve the problems such as restricting the application of fructus aglycone, poor medicinal effect of fructus aglycone, increasing blood cell level and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 liposome of the present invention
[0029] Prescription 1 (A): Burnet aglycone 1mg, HSPC 1mg, DSPE-PEG 2000 10mg, cholesterol 10mg, glucose 1mg;
[0030] Prescription 2 (B): Burnet aglycone 1mg, HSPC 5mg, DSPE-PEG 2000 30mg, cholesterol 3mg, sucrose 3mg;
[0031] Prescription 3 (C): Burnet aglycone 2mg, HSPC 5mg, DSPE-PEG 2000 20mg, cholesterol 10mg, trehalose 3mg;
[0032] Prescription 4 (D): Burnet aglycone 5mg, HSPC 10mg, DSPE-PEG 2000 30mg, cholesterol 30mg, fructose 5mg;
[0033] Prescription 5 (E): 7mg of Sanyu aglycone, 10mg of HSPC, 40mg of DSPE-PEG 2000, 50mg of cholesterol, 5mg of mannose;
[0034] Prescription 6 (F): Burnet aglycone 10mg, HSPC 10mg, DSPE-PEG 2000 40mg, cholesterol 50mg, lactose 5mg.
[0035] Prescription 7 (G): 1 mg of eucalyptus aglycone, 20 mg of carrier material (HSPC: DSPE-PEG 2000: cholesterol = 5: 1: 1, ie 14 mg of HSPC, 3 mg of DSPE-PEG 2000, 3 mg of cholesterol), 5 mg of sucrose.
[0036] Preparatio...
experiment example 1
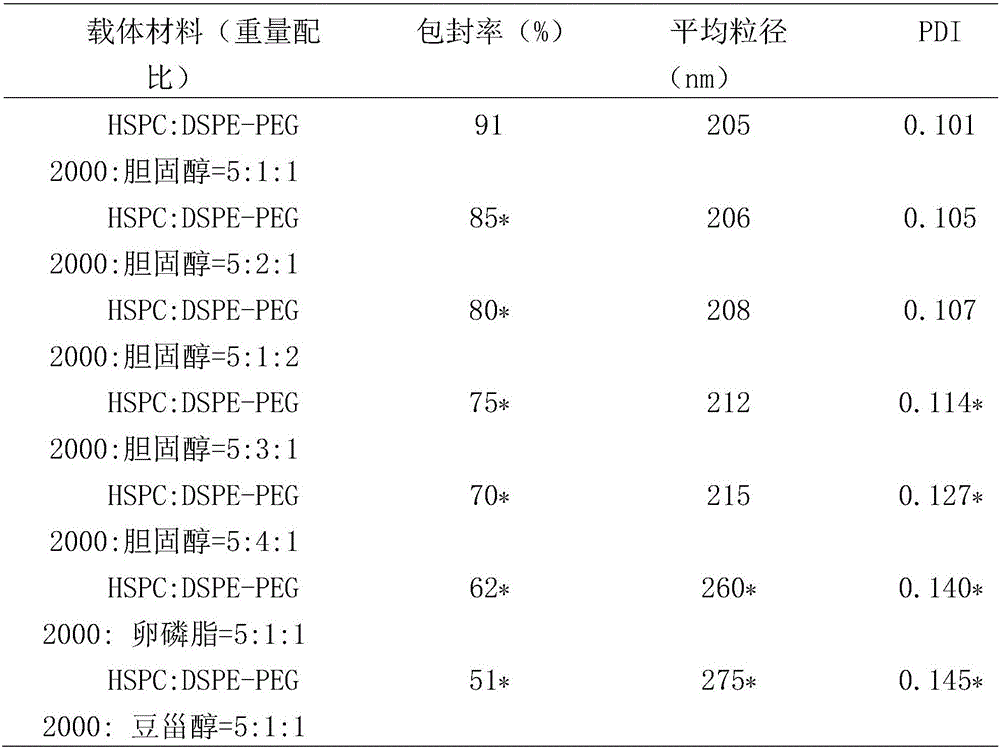
[0040] Experimental example 1 adopts the quality evaluation of different carrier materials to prepare buretin liposome
[0041] There are 7 experimental groups in this experiment.
[0042] Mix 1 mg of burnet aglycone with different carrier materials HSPC, DSPE-PEG 2000, cholesterol, lecithin, and stigmasterol mixed lipids according to the mass ratio of 1:20, dissolve in ethanol, remove ethanol by rotary evaporation under reduced pressure, and add 5 mg Sucrose and water, so that the concentration of eucalyptin in the liposome suspension is 0.2 mg / mL, high-pressure homogenization under 1000 bar pressure for 4 times, and sterilized by filtration with a 0.22 μm microporous membrane. The encapsulation efficiency, particle size distribution and dispersion index (PDI) of burnet aglycone in liposomes were measured, and the results are shown in Table 1.
[0043] Table 1 Quality Evaluation of Burnet Aglycone Liposomes
[0044]
[0045] Note: Compared with HSPC:DSPE-PEG 2000:cholest...
experiment example 2
[0049]The influence of experimental example 2 carrier material dosages on the quality of burnet aglycone liposome
[0050] There are 7 experimental groups in this experiment.
[0051] Weigh burnet aglycone and total lipid (HSPC:DSPE-PEG2000:cholesterol=5:1:1) according to the mass ratio shown in Table 2 respectively (fixed burnet aglycone mass is 1mg, total lipid mass varies with Ratio change), dissolved in ethanol, removed by rotary evaporation under reduced pressure, added 5mg of sucrose and water, so that the concentration of eucalyptin in the liposome suspension was 0.2mg / mL, high pressure homogenization under 1000bar pressure 4 times, Sterilize by filtration with 0.22μm microporous membrane. The encapsulation efficiency, average particle size and dispersion index (PDI) of burnet aglycone in liposomes were measured, and the results are shown in Table 2.
[0052] Table 2 Quality Evaluation of Burnet Aglycone Liposomes
[0053]
[0054] Note: Compared with the 1:20 gro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com