Synthetic stent for promoting growth of bone cells and making method thereof
A technology of bone cells and composite materials, which is applied in the field of synthetic scaffolds promoting bone cell growth and its preparation, can solve the problems of slow blood vessel growth, general prognosis, and slow cell growth speed, and achieve high response repeatability, easy post-processing, The effect of the simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A kind of preparation method of the synthetic support that promotes bone cell growth of the present invention, comprises the following steps:
[0045]First, add 2g of chitosan into 100ml of acetic acid with a volume ratio of 2%, and stir to prepare chitosan acetic acid colloid; Look, it may actually be more than 72mg) Salvianolic acid B was dissolved in 1mL ethanol and added to the acetic acid of chitosan and stirred evenly, then the Ca(NO 3 ) 2 、KH 2 PO 4 Add to the mixed solution and stir for a long time to prepare salvianolic acid B-chitosan / hydroxyapatite precursor solution, wherein the molar ratio of Ca / P is 5:3, and the final mass of chitosan / hydroxyapatite The ratio is about 1:2;
[0046] Then, the precursor solution was stirred for 4-5 hours and then centrifuged to remove air bubbles; then the solution was poured into a plastic mold, placed in an environment of 4°C for 2 hours, and in an environment of -10°C for 3 hours to freeze the solution into a solid (o...
Embodiment 1
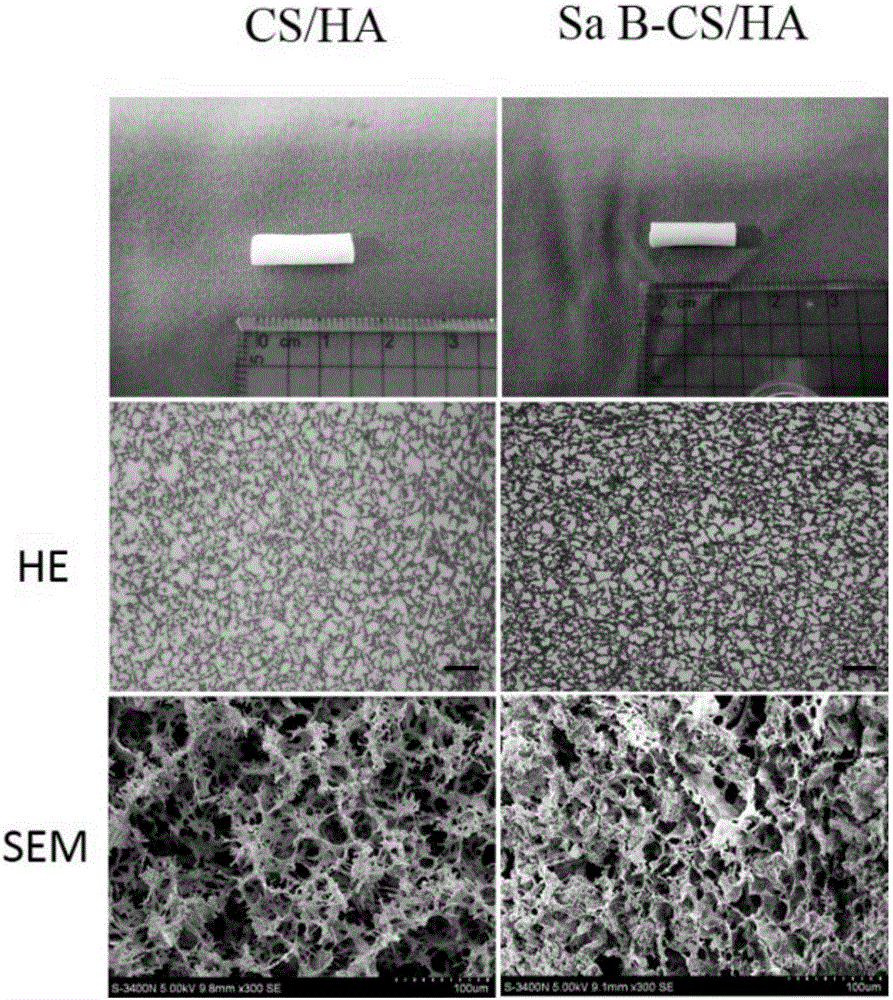
[0049] Salvianolic acid B (Sal B) (molecular formula: C 36 h 30 o 16 ,, molecular weight: 718.6) was purchased from sigma company (Tianjin, China); chitosan (CS) (molecular weight = 2.5 × 105, deacetylation degree ≥ 90%, viscosity > 100cps, biomedical grade) was purchased from Pioneer Biotechnology Company (Xi'an, China). First, 2g of chitosan was added to 100ml of 2% (V / V) acetic acid, and stirred for 20min to prepare a CS solution. Dissolve 72 mg of salvianolic acid B in 1 mL of ethanol and add it to the CS solution and stir for 2 h, then Ca(NO 3 ) 2 、KH 2 PO 4 (molar ratio Ca / P=1.67) was added to the mixed solution to prepare a Sal B-CS / HA precursor solution (the final mass ratio of CS / HA was 1 / 2). Then, the precursor solution was homogenized for 4 h and centrifuged for 10 min to remove air bubbles. The solution was poured into a plastic mold (diameter 4.0mm, length 20mm), placed at 4°C for 2h, -10°C for 3h, and then the solution was freeze-dried for 24h. After ful...
Embodiment 2
[0051] Salvianolic acid B (Sal B) (molecular formula: C 36 h 30 o 16 ,, molecular weight: 718.6) was purchased from sigma (Tianjin, China); chitosan (CS) (molecular weight = 2.5 × 105, degree of deacetylation ≥ 90%, viscosity > 100 cps, biomedical grade) was purchased from Pioneer Biotechnology Company ( Xi'an, China). First, 2g of chitosan was added to 100ml of 2% (V / V) acetic acid, and stirred for 20min to prepare a CS solution. Dissolve 48 mg of salvianolic acid B in 1 mL of ethanol and add it to the CS solution and stir for 2 h, then Ca(NO 3 ) 2 、KH 2 PO 4 (molar ratio Ca / P=1.67) was added to the mixed solution to prepare a Sal B-CS / HA precursor solution (the final mass ratio of CS / HA was 1 / 2). Then, the precursor solution was homogenized for 5 h and centrifuged for 10 min to remove air bubbles. The solution was poured into a plastic mold (diameter 4.0mm, length 20mm), placed at 4°C for 2h, -10°C for 3h, and then the solution was freeze-dried for 24h. After being ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com