Hypericin synthesis method
A technology of hypericin and a synthesis method is applied in chemical instruments and methods, preparation of organic compounds, preparation of quinones, etc., can solve the problems of inability to large-scale production, low yield and the like, and achieves low cost, high yield, The effect of a short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
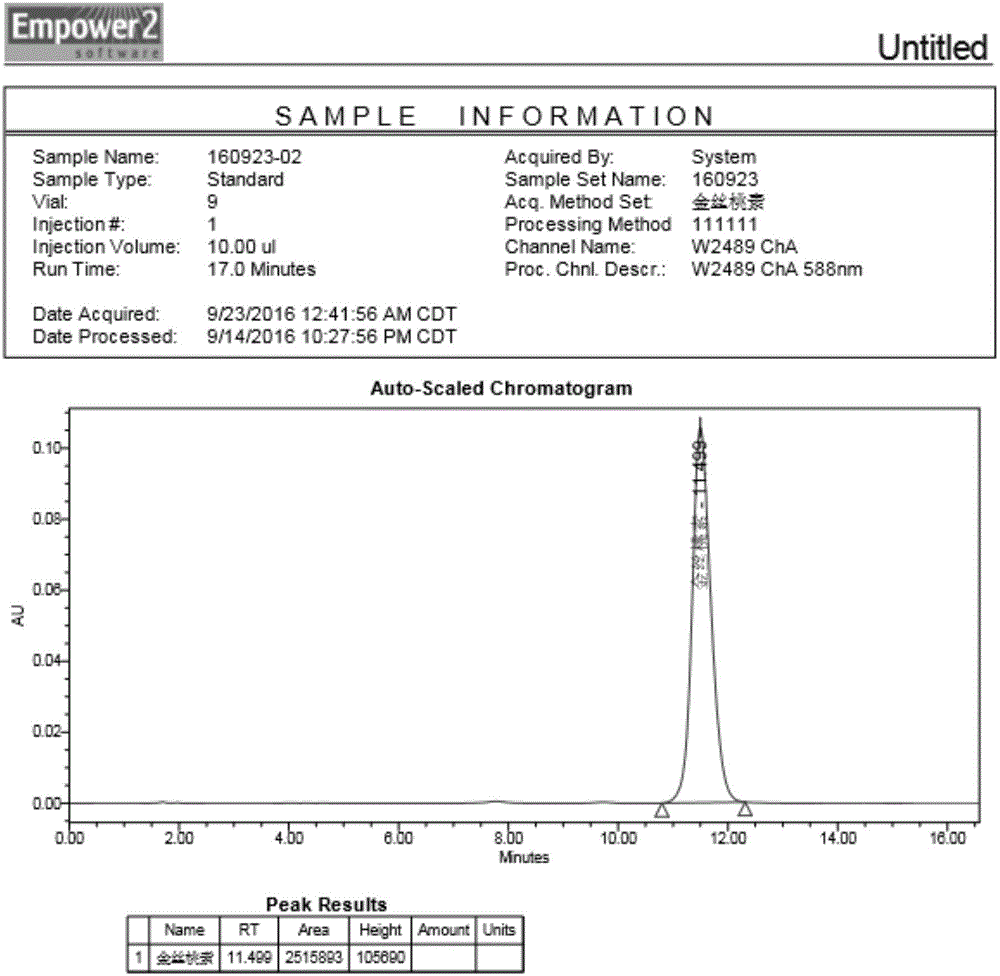
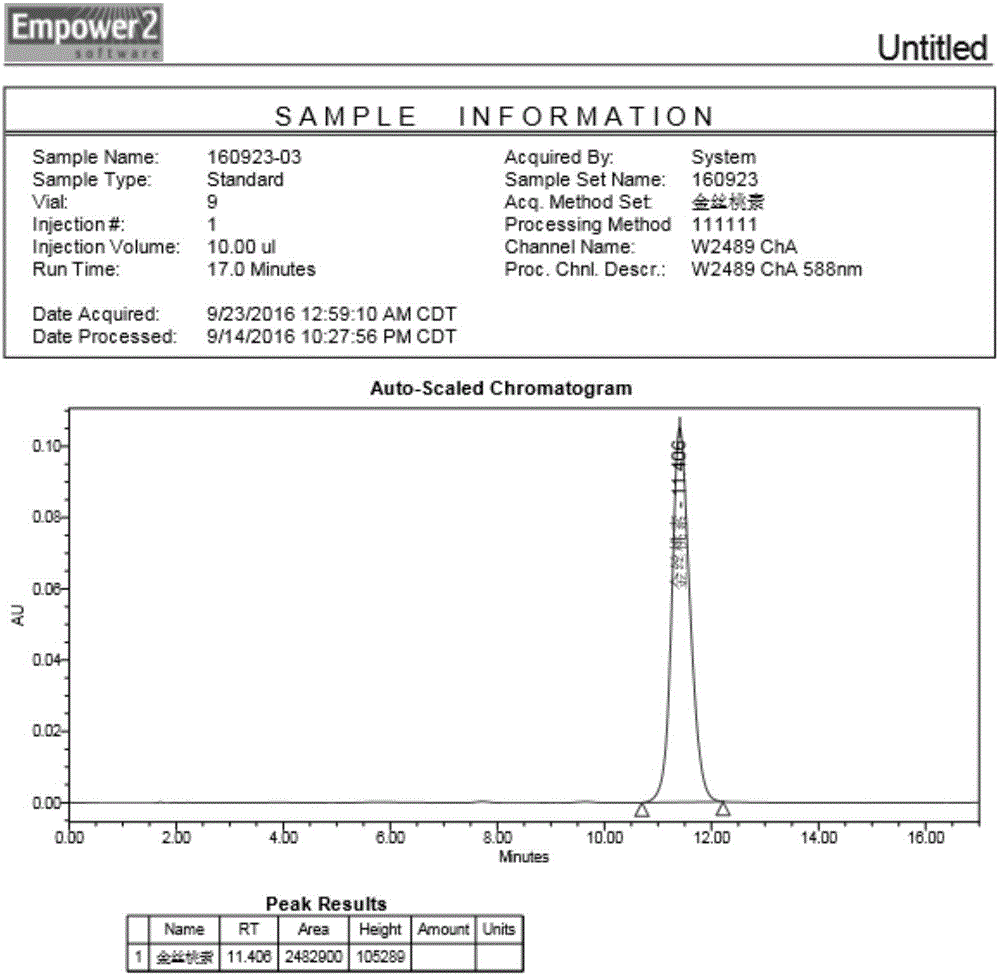
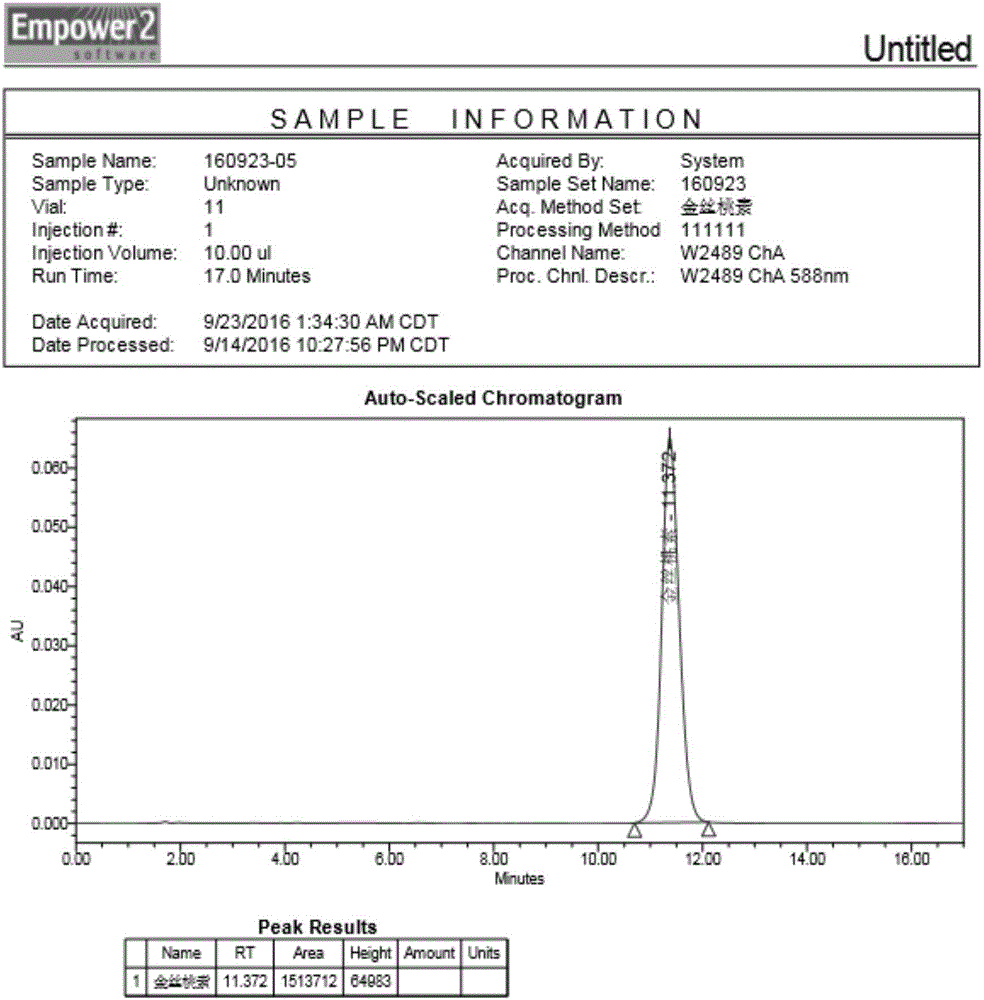
Image
Examples
Embodiment 1
[0032] (1) Put 30kg of emodin, 1500kg of glacial acetic acid, and 100kg of stannous chloride dihydrate into a 2-ton reaction kettle. 283kg of concentrated hydrochloric acid was added and reacted for two hours. Cool down to below 20 degrees, plate and frame filter and wash with water until neutral. Dried to obtain 27 kg of emodin enone.
[0033] (2) 30 kg of emodin anthrone, 600 kg of pyridine, 51.6 kg of piperidine, 60 kg of pyridine nitrogen oxide, and 3 kg of ferrous sulfate are dropped into 1 ton of reactor, and after adding, reflux reaction is carried out for 3 hours, and pyridine is reclaimed under reduced pressure to a small volume. After cooling, add dilute hydrochloric acid to neutralize the pH value at about 5-6, centrifuge or plate-and-frame filter and wash with water until neutral to obtain prohypericin, and dry at 50 degrees to obtain 30 kg for later use.
[0034] (3) 15kg of former hypericin is dissolved in 1500L concentration and is that the sodium hydroxide so...
Embodiment 2
[0036] (1) Emodin 20kg glacial acetic acid 1000kg stannous chloride dihydrate 68kg is thrown into 2 tons of reactor, after adding, the temperature is raised to reflux, then slowly add 55.3kg concentrated hydrochloric acid and react for one hour after adding, then slowly add 188.7 kg concentrated hydrochloric acid. The reaction was completed for two hours. Cool down to below 20 degrees, plate and frame filter and wash with water until neutral. Dry to obtain 19 kilograms of emodin enquinone.
[0037] (2) 20 kg of emodin anthrone, 400 kg of pyridine, 34.4 kg of piperidine, 40 kg of pyridine nitrogen oxide, and 2 kg of ferrous sulfate are put into 1 ton of reactor, and after adding, reflux reaction is carried out for 3 hours, and pyridine is reclaimed under reduced pressure to a small volume. After cooling, add dilute hydrochloric acid to neutralize the pH value at about 5-6, centrifuge or plate and frame filter and wash with water until neutral to obtain prohypericin, and dry a...
Embodiment 3
[0040](1) Emodin 10kg glacial acetic acid 450kg stannous chloride dihydrate 35kg into a 1-ton reaction kettle, after the addition, the temperature was raised to reflux, then slowly added dropwise 28kg concentrated hydrochloric acid, reacted for one hour after the addition, and then slowly added dropwise 94kg Concentrated hydrochloric acid. The reaction was completed for two hours. Cool down to below 20 degrees, plate and frame filter and wash with water until neutral. Dried to obtain 9 kg of emodin enone.
[0041] (2) 9 kg of emodin anthrone, 200 kg of pyridine, 17.2 kg of piperidine, 20 kg of pyridine nitrogen oxide, and 1 kg of ferrous sulfate are put into a 1 ton reactor, and after the addition, the reflux reaction is carried out for 3 hours, and the pyridine is recovered under reduced pressure to a small volume. After cooling, add dilute hydrochloric acid to neutralize the pH value at about 5-6, centrifuge or plate and frame filter and wash with water until neutral to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com