Method for producing low-acetaldehyde polyester product
A production method and low acetaldehyde technology, applied in the field of low acetaldehyde polyester products, can solve the problem of high residual acetaldehyde
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
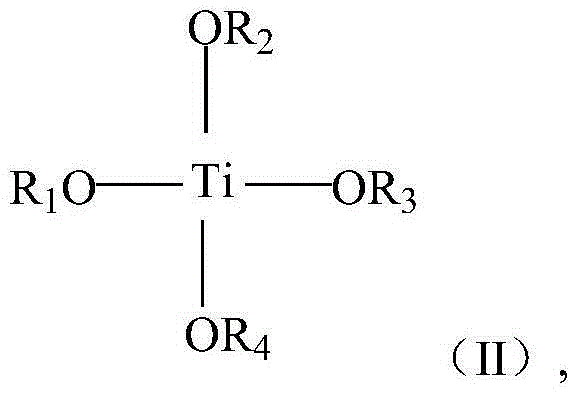
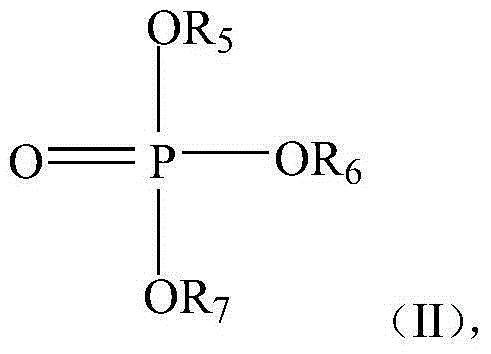
[0072] Preparation of Catalyst A
[0073] Add 12.4 grams (0.2 mol) of ethylene glycol in the reactor that stirrer, condenser and thermometer are equipped with, slowly drop into reactor 28.4 grams (0.1 mol) tetraisopropyl titanate, separate out white precipitate, After reacting at 70°C for 2 hours, the product was centrifuged, and the residue was washed with distilled water three times, and the product was vacuum-dried at 70°C to obtain a white powdery substance.
[0074] The white powder material after drying is placed in the reactor that has agitator, condenser and thermometer, adds ethylene glycol 50 grams, 48 grams of 25wt% sodium hydroxide aqueous solution (0.3 mole), magnesium acetate 42.8 grams ( 0.2 moles), 42 grams (0.2 moles) of citric acid monohydrate, and 42 grams (0.3 moles) of trimethyl phosphate were reacted for 2 hours at a reaction temperature of 150° C. to obtain a nearly colorless homogeneous liquid, which was catalyst A.
[0075] Preparation of polyester ...
Embodiment 2
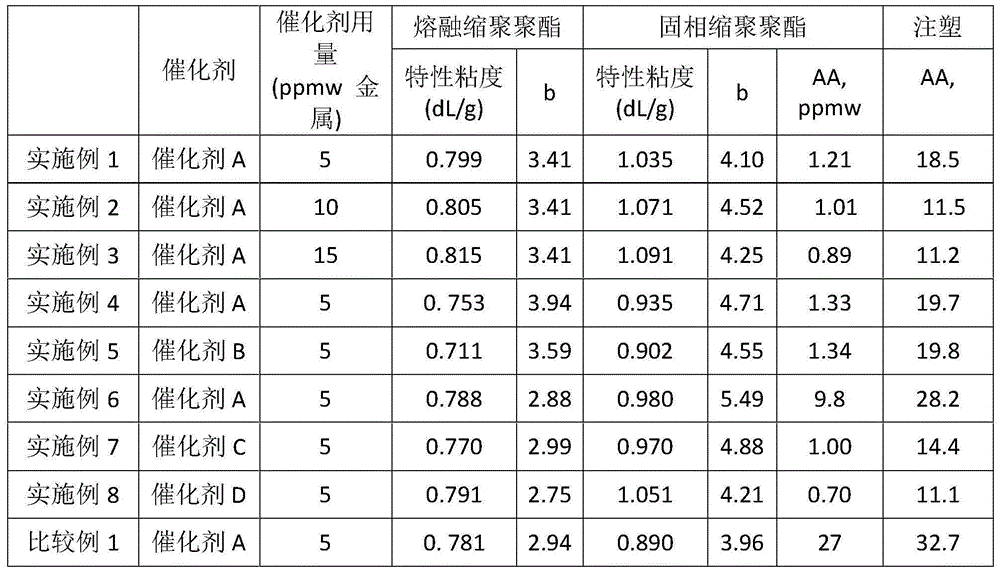
[0082] Except that catalyst A (based on the amount of polyester produced, the weight of titanium atoms was changed to 10ppmw), the same method as in Example 1 was used to carry out the preparation of polyester and the solid-phase polymerization of polyester.
[0083] The test results are shown in Table 1.
Embodiment 3
[0085] Except that catalyst A (based on the amount of polyester produced, the weight of titanium atoms was changed to 15 ppmw), the same method as in Example 1 was used to carry out the preparation of polyester and the solid-phase polymerization of polyester.
[0086] The test results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com