Building method for acute hyperuricemia animal model
A technology of hyperuricemia and construction method, which is applied in the field of construction of animal models of acute hyperuricemia, can solve the problems of unsuitable PNP enzyme inhibitors for reducing uric acid, and achieve good reproducibility and simple construction method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Experimental reagents
[0020] Uric acid (UA) assay kit: 50T / 48 samples, batch number: 20160701, Nanjing Jiancheng Bioengineering Research Institute; sodium chloride injection: 250ml, 2.25g, batch number: A140829G2, Kunming Nanjiang Pharmaceutical Co., Ltd. Inosine: 14125-10G, Lot #019K1124V, CAS: 58-63-9, Sigma.
[0021] 2. Experimental animals
[0022] Male rhesus monkey, weighing 6-8kg, production license number: SCXK (Dian) 2011-0005, use license number: SYXK (Dian) K2014-007.
[0023] 3. Experimental equipment
[0024] SYNERGY / HT multifunctional microplate reader, BioTek; MiniSpin centrifuge, eppendorf; NewClassic MFMS105 analytical balance, METTLER TOLEDO.
[0025] 4. Experimental method
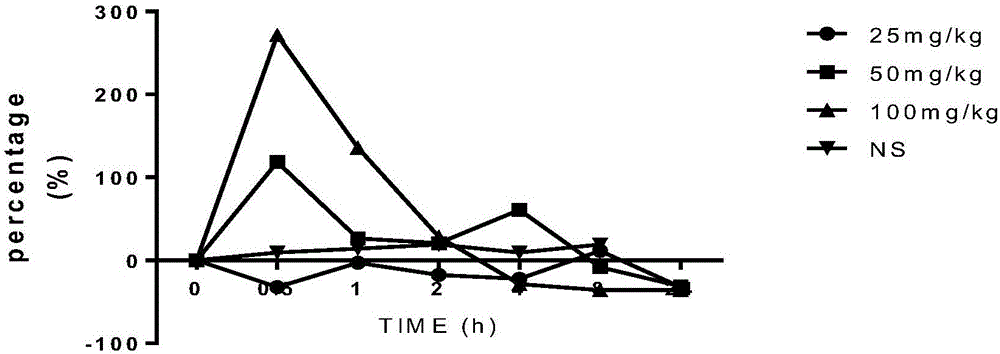
[0026] Four rhesus monkeys were divided into four groups: model group and normal saline control group. The model group was intraperitoneally given different doses of inosine solution to create models. High dose group (100mg / kg), middle dose group (50mg / kg), low dose gro...
Embodiment 2
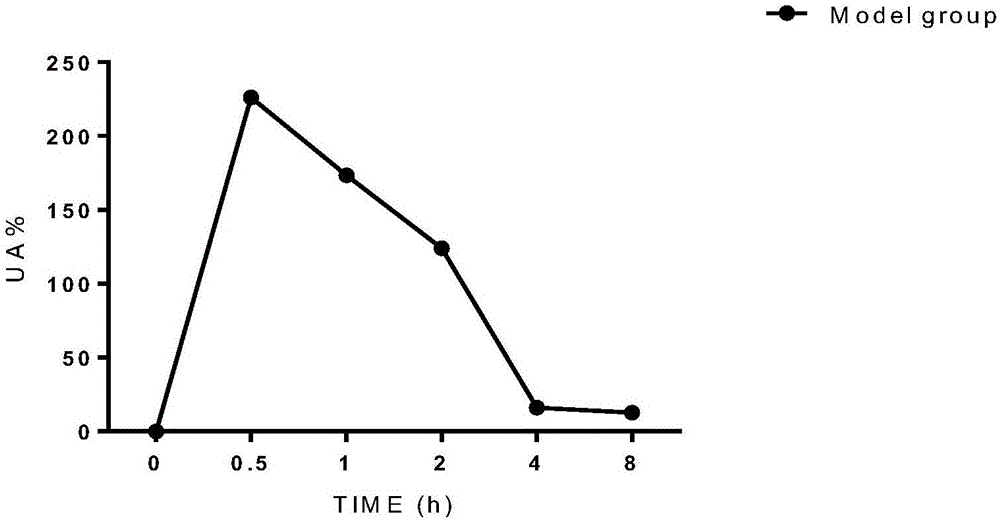
[0033] After a time interval of two weeks, the 4 rhesus monkeys used in the experimental group of Example 1 were used, 2 new male rhesus monkeys were added, and 6 monkeys were used to build a model, and the blood uric acid level was used as a control. The dose of inosine is (100mg / kg), intraperitoneally injected, and the administration volume is 2ml / kg. About 2ml of blood was taken at different time points, and the blood uric acid value was compared at different time points. The time points were set as: 0h (that is, blank control blood was taken before modeling), 0.5h, 1h, 2h, 4h, and 8h after modeling. After the blood samples were left at room temperature for 30 minutes, they were centrifuged at 4000r / min for 10 minutes, and the serum was taken and immediately frozen in a -80°C refrigerator. After the serum was collected at all time points, the uric acid value was determined according to the kit method (phosphotungstic acid method). The results are shown in Table 2, figure ...
Embodiment 3
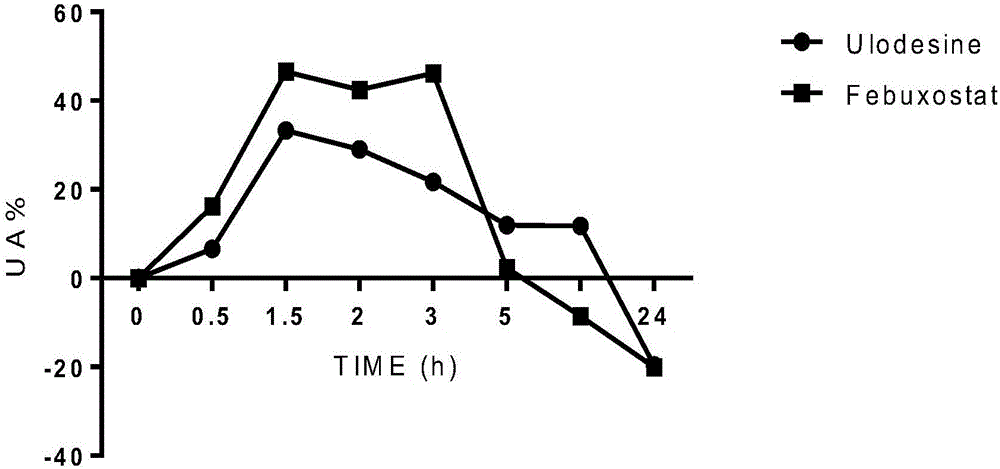
[0039] Six male rhesus monkeys were divided into two drug groups. 3 only for Ulodesine group, 3 only for Febuxostat group. Drugs were given 1 hour before modeling. The dose of Ulodesine was 2 mg / kg (equivalent to clinical human dose), and the dose of Febuxostat was 2 mg / kg (equivalent to clinical human dose). The dosage of the modeling agent inosine is (100mg / kg), injected intraperitoneally, and the administration volume is 2ml / kg. Take about 2ml of blood at different time points, and compare the blood uric acid values at different time points. 4h, 8h, and 24h after administration. After the blood samples were left at room temperature for 30 minutes, they were centrifuged at 4000r / min for 10 minutes, and the serum was taken and immediately frozen in a -80°C refrigerator. After the serum was collected at all time points, the uric acid value was determined according to the kit method (phosphotungstic acid method). The results are shown in Table 3, image 3 and Figure 4 ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com