Medical device implanted in vivo and manufacturing method of medical device
A technology of medical devices and manufacturing methods, which is applied in the field of medical devices, can solve the problems of protective film easy to fall off, protective effect, loss, etc., and achieve the effect of not easy to fall off and tight connection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] An implantable medical device comprising:
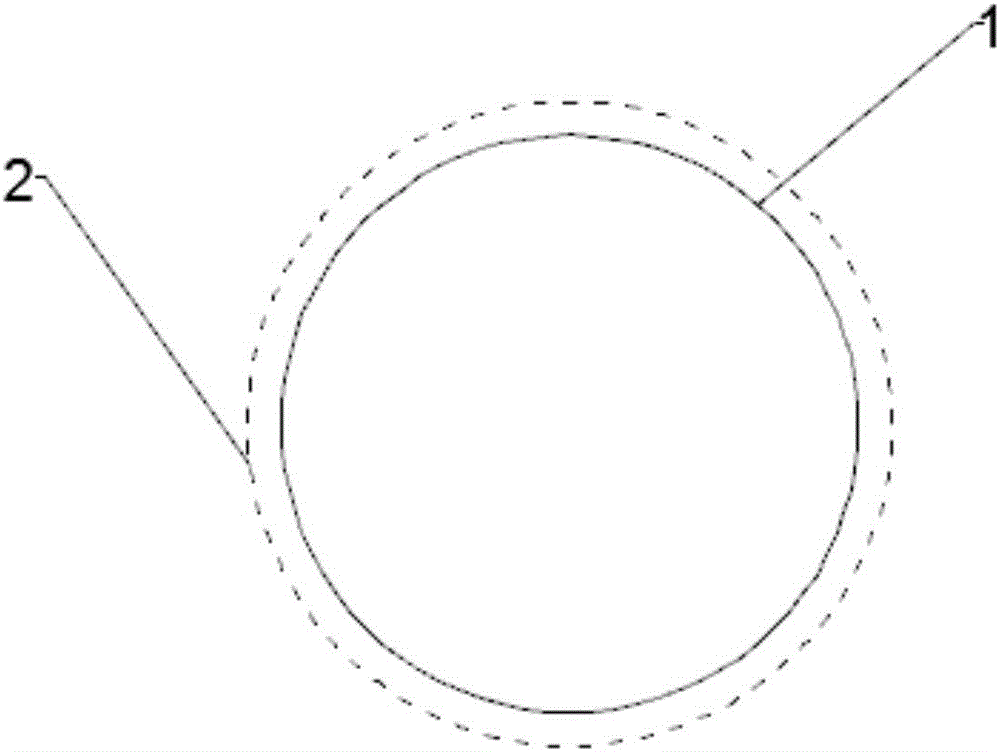
[0035] Ontology 1;
[0036] A protective film 2, covering the surface of the body 1, for reducing the degradation rate of the body 1;
[0037] The surface of the body 1 has a certain roughness, and the protective film 2 is directly coated on the surface of the body 1 . In the present invention, the surface of the main body 1 is treated to make it have a certain roughness, so that the protective film 2 can be better attached to the main body 1, so that the protective film 2 is not easy to fall off.
[0038] The main body 1 is made of magnesium or magnesium alloy material, and the main body 1 has a crown and bridge ribs, and the protective film 2 covers the crown and bridge ribs. Since the crown and the tendons are the key protection positions, covering them with the protective film 2 can slow down the degradation rate of the weak positions.
[0039] The thickness of the protective film 2 is 10nm-1000nm.
[0040] The protect...
Embodiment 2
[0043] A method for manufacturing a medical device implantable inside a living body, comprising the following steps:
[0044] Step 1, preparing the body 1;
[0045] Step 2, performing surface treatment so that the surface of the body 1 has a certain roughness;
[0046] Step 3, coating the protective protective film 2.
[0047] In the present invention, the surface of the main body 1 is treated to make it have a certain roughness, so that the protective film 2 can be better attached to the main body 1, so that the protective film 2 is not easy to fall off.
[0048] The body 1 is prepared by laser cutting or wire welding.
[0049] The surface treatment is surface sandblasting, the number of mesh of the abrasive used is 200 mesh, the sandblasting power is 200w, the distance between the nozzle and the surface of the body 1 is 1mm, the moving speed of the body 1 is 2mm / s, and the rotation speed is 2mm / s. 0.5 turns / s.
[0050] The surface treatment is plasma treatment with a pow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com